Chemistry of Diamonds
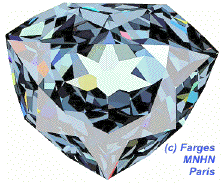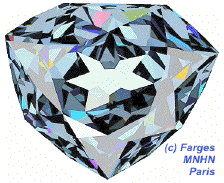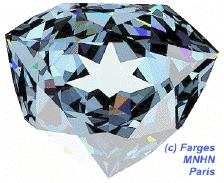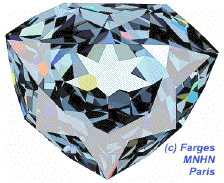 This simulation of the hope diamond[1] illustrates how light reflects off multiple facets, making the gem appear shiny.
This simulation of the hope diamond[1] illustrates how light reflects off multiple facets, making the gem appear shiny.
Diamond and graphite are examples of allotropes, where the same element forms two distinct crystalline forms. Diamond is one of the hardest known substances, prized for the transparent and highly reflective crystals that make it sparkle. In addition to making fine gemstones, diamond is also used industrially for cutting, grinding, sawing, and drawing wire.
 A diamond blade is used to prepare thin sections for microscopy[2]
A diamond blade is used to prepare thin sections for microscopy[2]
Graphite, on the other hand, is a soft, black substance used to make pencils.
Diamonds and graphite are both non-metals made exclusively of carbon atoms.
Diamond Crystal Structure
The hardness and density of diamonds can be explained by their crystal structure. Each carbon atom is surrounded by four other carbon atoms in a regular tetrahedron. Each of these carbon atoms is then attached to three other carbon atoms (plus the original atom), and that pattern continues to form a single, giant molecule held together by covalent bonds. In order to break the crystal, multiple bonds must be broken.
 Structure of diamond. Public domain image.
Structure of diamond. Public domain image.
Graphite Crystal Structure
Graphite forms in layers. Each carbon atom forms two single bonds and one double bond with the three closest neighboring carbon atoms, giving a resonance structure with 2/3 single bond character and 1/3 double bond character. The distance between these bonds within a layer is about half the distance between layers, so graphite resembles a bunch of long, flat molecules stacked in a pile. The individual molecules cannot be separated, but the layers are easy to separate, explaining the soft, lubricating nature of these crystals.
 Structure of graphite. Public domain image.
Structure of graphite. Public domain image.
 Chemical structures and photographs of diamond and graphite[4]
Chemical structures and photographs of diamond and graphite[4]
References
- Francoisfarges, . Diamantbleu.gif. Retrieved from https://commons.wikimedia.org/wiki/File:Diamantbleu.gif
- microscopyTraining, . Using a 'Histo' Diamond Knife for Sectioning. Retrieved from https://www.youtube.com/watch?v=IavGHsEID-A
- Goldstein, J. Rainy Day Activity - Giant Pencil. Retrieved from https://www.youtube.com/watch?v=n0lSmUH3u18
- ltub, . Diamond and Graphite. Retrieved from https://commons.wikimedia.org/wiki/File:Diamond_and_graphite.jpg