Optical Isomerism
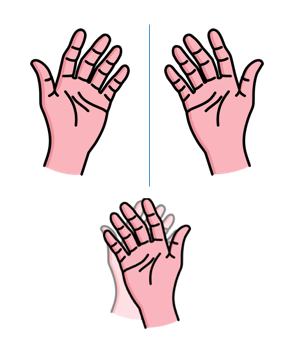
Study your hands for a moment. Nearly everything about these two structures is identical. You have the same number of fingers on each hand, and they are connected in the same order. Your hands are the same size. The skin on both palms reacts the same way when you touch the handle of a hot pan. (probably)
If you hold both hands up in front of you, they are mirror images of each other, with the angles and dimensions of the left hand repeated perfectly on the right side. However, if you place your right hand on top of your left hand, they no longer look identical. If two molecules are optical isomers of each other, their relationship is analogous to the relationship between the left and right hands.
Importance
Optical isomers share all of their physical and chemical properties except one: the direction that they rotate plane-polarized light. While this may seem like a trivial difference, optical isomerism can have a major effect on the behavior of a molecule. Many drugs contain both optical isomers, and while the chemical structure is the same, the enantiomers are metabolized differently in the body, cause different side effects, and vary in potency. One isomer may be beneficial, while the other may be deadly. As a result, studying optical isomerism is important in drug development."Knowledge of isomerism has helped us in introducing safer and more effective drug alternatives." [1]
Isomerism Defined
Isomers are two molecules that have the same atomic composition, but are not identical.
Many organic compounds form constitutional isomers, which are also called structural isomers. They have the same empirical formula, but the atoms are connected in a different way. For example, \(C_2H_6O\) is the empirical formula for both ethanol and dimethyl ether. These two compounds have different melting points, refractive indices, and specific gravities, and as a result they have different industrial uses as well. Ethanol is a liquid at room temperature, and is used as an industrial solvent and the alcohol in alcoholic beverages. Dimethyl ether is a gas at room temperature and can be used as a refrigerant.
Configurational isomers have identical atomic composition and an identical bonding arrangement, but the molecules are arranged differently in space and cannot interconvert to become identical. Optical isomers, also called enantiomers, fit into this category.
The third type of isomer is the conformational isomer. Conformational isomers are similar to configurational isomers, in that they have the same atoms bonded together in the same order. However, conformational isomers can interconvert. They contain a single bond (a sigma bond) that allows the atoms to rotate.
Enantiomers
Enantiomers, as mentioned earlier, are mirror images that cannot be superimposed on one another. These molecules are also referred to as chiral molecules. Chirality can be shown in 2-D drawings using the Wedge-Dash Notation or Newman Projections.
The only way in which chiral molecules differ is the direction that they rotate plane polarized light. In plane polarized light, all the waves are moving parallel to each other. Two enantiomers will rotate the direction the light waves are moving by the same magnitude, but in opposite directions. A sample containing only one enantiomer is enantiopure, while a sample containing both is called a racemic mixture.
Enantiomers are described as having a handedness depending on the direction they rotate plane-polarized light. Dextrorotatory isomers rotate the light to the right. Dextra is Latin for "right". The Latin word Levorotatory Laevus can be translated from Latin not only as left, but also as ungainly, bumbling, or foolish.
| Dextrorotatory | Levorotatory |
| + | - |
| rotates light clockwise | rotates light counterclockwise |
Enantiomers can be labelled using different conventions: R/S, D/L, +/-, but the only convention that tells us what direction they rotate plane-polarized light is +/-. You can't say whether a chiral compound is the + or - enantiomer just by looking at its structure. You can only tell that by actually doing the experiment and seeing how they rotate plane-polarized light. You can, however, use the R/S and the D/L conventions when you only know the structure. The R/S convention is the most frequently used. The R enantiomer of a given compound can be - just as well as it can be +. You don't know it is + or - until actually checking it with plane-polarized light. You can also use d for the +(dextrorotatory) enantiomer and l for the levorotatory one, but it's best to only use +/- and R/S to tell them apart, because d/l is confusing, for its name is similar to D/L (but D/L is another convention that you can use when you only know the structure).
Despite the names, the levorotatory isomer is not always undesirable. Many drugs are synthesized as racemic mixtures because they can be difficult to purify, but often the two enantiomers are metabolized differently and act differently in the body, with one causing more of the desirable effects and the other potentially causing more adverse effects. For example, the antidepressant citalopram is a racemic mixture, while escitalopram contains only the s-enantiomer. A patient only has to take half as much escitalopram to get the same effect (a standard dose is 5-10 mg, compared to 10-20 mg for citalopram).
Amino acids also form enantiomers (for example, there is an l-lysine and a d-lysine), but only the levorotatory isomer is used by cells to make proteins.
![Researchers at NASA think asteroids might hold clues as to why life only uses "left-handed" amino acids. [5]](https://ds055uzetaobb.cloudfront.net/brioche/uploads/H67UtSVDmG-317849main_pnas_extra_jpg.jpg?width=1200) Researchers at NASA think asteroids might hold clues as to why life only uses "left-handed" amino acids. [5]
Researchers at NASA think asteroids might hold clues as to why life only uses "left-handed" amino acids. [5]
Thalidomide
Thalidomide is a well-known example of a racemic mixture. This drug was developed in the first half of the twentieth century as a sedative, and was also very effective for treating the morning sickness and nausea that many women experience during pregnancy. Thalidomide was very popular in Europe in the 1950's, but its popularity coincided with an increasing number of children experiencing debilitating birth defects. The most famous side effect of thalidomide is phocomelia, a condition where limbs are either missing entirely or are very short, leaving the hands or feet to develop close to the trunk of the body. Deafness and other bone-related deformities are also seen in thalidomide victims.
![A young child with thalidomide-related phocomelia [3]](https://ds055uzetaobb.cloudfront.net/brioche/uploads/sZN1b91GL0-terry_wiles_2a.jpg?width=1200) A young child with thalidomide-related phocomelia [3]
A young child with thalidomide-related phocomelia [3]
![Malformation of the feet, attributed to thalidomide use [4]](https://ds055uzetaobb.cloudfront.net/brioche/uploads/GoHNT0qea3-2241322031_f5f56a7799_o.jpg?width=1200) Malformation of the feet, attributed to thalidomide use [4]
Malformation of the feet, attributed to thalidomide use [4]
The drug was eventually pulled from the market, after at least 4,000 children had been affected [2]. Later, studies done in mice suggested that the R enantiomer of thalidomide was pharmacologically active as a sedative, while the S enantiomer was responsible for the birth defects.
Thalidomide is still available and is used to treat cancer, especially multiple myeloma. However, the drug carries a strict warning against use in pregnant women.
References
[1] Chhabra, Naveen, MadanL Aseri, and Deepak Padmanabhan. "A Review Of Drug Isomerism And Its Significance". Int J App Basic Med Res 3.1 (2013): 16. Accessed from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3678675/ February 29, 2016.
[2] National Institutes of Health. "Changing the Face of Medicine." Accessed from https://www.nlm.nih.gov/changingthefaceofmedicine/physicians/biography_182.html February 29, 2016.
[3] Image from http://www.terrywiles.20m.com/ under Creative Commons licensing for reuse and modification.
[4] Image from https://www.flickr.com/photos/22719239@N04 under Creative Commons licensing for reuse and modification.
[5] Image from http://www.nasa.gov/centers/goddard/news/topstory/2009/lefthandlife.html under Creative Commons licensing for reuse and modification.
[6] Image from http://publicdomainvectors.org/en/free-clipart/Vector-image-of-open-your-palms/24859.html under Creative Commons licensing for reuse and modification.