Phase Changes
Phase changes are physical changes that take place when matter changes energy states, but chemical bonds are not broken or formed.
For example, the physical properties of ice, liquid water, and steam are quite different even though they are all \(\ce{H2O}\) and there is no difference in the molecular structure of the substances.
The phase of a substance depends on the amount of energy the atoms contain. All atoms are in motion. The higher the temperature of an atom, the faster the motion. A solid is vibrating in place, although your eyes cannot detect this. A liquid form allows atoms to roll around each other but not bounce out of their container. A gas is of such a high energy state that the molecules are literally bouncing off the container and taking up as much space as they are allowed. The higher the energy level, the faster and farther apart the atoms move.
Contents
Phases Defined
![Fluorescent lamps use low pressures to turn gases into plasma. In this lamp, the gas is argon. [1]](https://ds055uzetaobb.cloudfront.net/brioche/uploads/mMz4E8u498-bell_jar_35_with_argon_plasma.JPG?width=1200)
Solids are usually crystalline, meaning they have a repeating, ordered 3-dimensional structure. The two most common types of crystals are metallic crystals and ionic crystals. Metallic solids consist of metal atoms packed tightly together. They tend to have high melting and boiling points. Metals often conduct electricity well, because their outer electrons move freely from atom to atom. Ionic solids are aggregates of positively and negatively charged ions. Table salt, NaCl, is one common example. Ionic crystals also have high melting and boiling points, but they are usually poor conductors of electricity, because the compounds have strong electrostatic interactions between the positively and negatively charged ions.
The particles in liquids are close together, making this phase difficult to compress or expand, but the particles are not fixed like in a solid. They can slide past one another, making it possible for liquids to change shape and to mix with another liquid or even a material in a different phase. This attribute is important, because it allows liquids to form solutions. These uniform mixtures of two or more substances are where many chemical reactions take place. The degree to which two liquids mix is called their miscibility. Oil and water are highly immiscible and their molecules tend to repel each other.
Gases are arguably the least complex phase of matter, because all gases exhibit similar behavior and follow the same laws such as the ideal gas laws, regardless of identity. Gas particles move very rapidly and are physically far apart from each other, so only weak intermolecular forces exist between the particles. These characteristics make gases fluid in their shape and volume and relatively easy to compress.
 Summary of Common Phases
Summary of Common Phases
Phase Equilibria
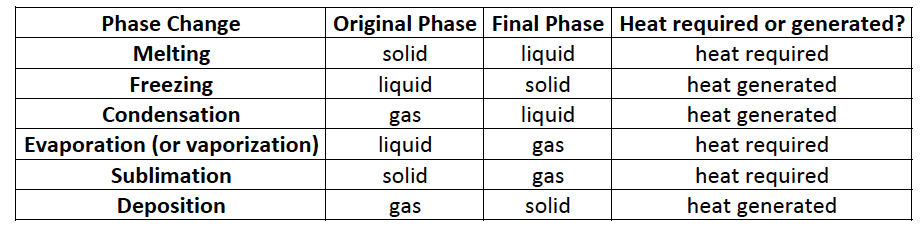 Summary of Phase Changes
Phase changes are reversible, and an equilibrium exists between phases at certain conditions. For example, the liquid-solid equilibrium point for water is at 1 atm and \(0^{\circ}C\). Under those conditions, an ice cube floating in a glass of water would be constantly undergoing phase changes (some of the ice is absorbing heat and melting and some of the water is releasing heat and freezing), but the relative proportion of solid to liquid remains the same.
Summary of Phase Changes
Phase changes are reversible, and an equilibrium exists between phases at certain conditions. For example, the liquid-solid equilibrium point for water is at 1 atm and \(0^{\circ}C\). Under those conditions, an ice cube floating in a glass of water would be constantly undergoing phase changes (some of the ice is absorbing heat and melting and some of the water is releasing heat and freezing), but the relative proportion of solid to liquid remains the same.
![Liquid carbon dioxide, also known as dry ice, undergoes sublimation. It is commonly used as a refrigerant. [2]](https://ds055uzetaobb.cloudfront.net/brioche/uploads/ApgaGH0mfb-dry_ice_in_cup.jpg?width=1200) Liquid carbon dioxide, also known as dry ice, undergoes sublimation. It is commonly used as a refrigerant. [2]
The temperature of a liquid is determined by the kinetic energy of its molecules. Some molecules might have enough energy to reach the liquid-gas equilibrium, where the liquid evaporates and enters the gas phase. When those higher energy particles enter the gas phase, it decreases the average temperature of the liquid, so evaporation is a cooling process. Another way to think of it is that heat is required to make evaporation happen. Sweating is one example of evaporation in action. The opposite of evaporation is condensation. When a gas condenses, it moves to a lower kinetic energy state and heat is generated as a result.
Liquid carbon dioxide, also known as dry ice, undergoes sublimation. It is commonly used as a refrigerant. [2]
The temperature of a liquid is determined by the kinetic energy of its molecules. Some molecules might have enough energy to reach the liquid-gas equilibrium, where the liquid evaporates and enters the gas phase. When those higher energy particles enter the gas phase, it decreases the average temperature of the liquid, so evaporation is a cooling process. Another way to think of it is that heat is required to make evaporation happen. Sweating is one example of evaporation in action. The opposite of evaporation is condensation. When a gas condenses, it moves to a lower kinetic energy state and heat is generated as a result.
Gases and solids can also exist in equilibrium, though examples are less common in everyday life. Solid moving directly to gas is called sublimation while the reverse is deposition.

A cup of warm water is suspended in a large pot of water held at a steady boil at atmospheric pressure. Will the water in the cup ever boil?
Assume that the pot never runs out of water and that the environment remains unchanged.
Phase Change Diagrams
![A generalized phase change diagram for a single substance. The solid green line shows the usual shape of a liquid-solid equilibrium, while the dotted green line shows the anomalous behavior of water. [3]](https://ds055uzetaobb.cloudfront.net/brioche/uploads/ncgYqtYA6h-screen-shot-2016-02-15-at-85718-am.png?width=1200) A generalized phase change diagram for a single substance. The solid green line shows the usual shape of a liquid-solid equilibrium, while the dotted green line shows the anomalous behavior of water. [3]
A generalized phase change diagram for a single substance. The solid green line shows the usual shape of a liquid-solid equilibrium, while the dotted green line shows the anomalous behavior of water. [3]
Phase change diagrams show what phase a substance will be in at a given temperature and pressure. The triple point is the only temperature and pressure at which all three phases can exist simultaneously.
Every substance has a critical temperature, which is the maximum temperature where it can exist as a liquid. Additionaly, each substance has a critical pressure, which is the minimum pressure needed to liquify a substance at its critical temperature. The point where the critical temperature and critical pressure meet is called, appropriately, the critical point. This is where the densities of the gas and liquid phases become equal. Beyond that point, the two phases are indistinguishable and are called a supercritical fluid.
Changing the amount of heat energy in a substance usually causes a temperature change. However, during a phase change, the temperature stays the same even though the heat energy changes. This energy is going into changing the phase and not into raising the temperature. That's why water doesn't get hotter while it boils. The temperature remains constant until the phase change is complete.
Phase Change and Latent Heat
Suppose we are given a sample of pure ice and we decide to heat it. We heat the ice with the help of an electric heater which supplies heat at a constant rate. Then, if we measure the temperature of the sample and plot its variation with time, we get a graph similar to the one shown below.

Specific Latent Heat
Not to be confused with Specific heat
The specific latent heat of a phase change is the energy required to completely change the phase of unit mass of the substance keeping the temperature constant. \(_\square\)
The unit of specific latent heat is \( \text{J kg}^{-1} .\)
The latent heat of vaporization of water is about \(2265 \text{ kJ kg}^{-1},\) and the latent heat of fusion is about \(334 \text{ kJ kg}^{-1}.\)
How much energy is required to melt \(5\text{ kg}\) of ice to water at \(0^{\circ} \text{C} \)?
The latent heat of fusion is \( 334 \text{ kJ kg}^{-1}\). This means that we require \(334 \text{ kJ}\) of energy to melt one kilogram of ice. Since we want to melt \(5\text{ kg}\) of ice, we will require \( 334k \times 5 = 1670k \) Joules of energy. \(_\square\)
References
[1] Image from https://commons.wikimedia.org/wiki/File:BellJar35withArgon_Plasma.JPG under Creative Commons licensing for reuse and modification.
[2] Image from https://commons.wikimedia.org/wiki/File:Dryicein_cup.jpg under Creative Commons licensing for reused and modification.
[3] Image from https://commons.wikimedia.org/wiki/File:Phase-diag.svg under Creative Commons licensing for reuse and modification.