Structural Isomerism
In organic chemistry the chemical formula doesn't give us the exact properties of the material as there can be many isomers for the same compound. Isomerism is defined as a phenomenon in which two or more organic compounds have the same chemical formula but have different structural formula along with different physical properties.
Structural Isomerism
Isomers which differ only in the arrangement of atoms within the molecule without any reference to the spatial arrangement (stereoisomerism) are known as structural isomers, and the same phenomenon is called structural isomerism.
So when two molecules have the same molecular formula but a different structural formula, they are said to exhibit structural Isomerism. Structural isomerism can be of the following types:
Chain Isomerism
In IUPAC nomenclature, a main chain is chosen taking many perspectives into consideration. The main chain determines the name and structure of the compound.
Chain Isomerism
Chain isomerism arises due to the difference in the arrangement of carbon chain within the molecule. In other words, if two compounds with the same molecular formula have a different main chain, then they exhibit chain isomerism.
This is how \(\ce{C6H14}\) exhibits chain isomerism. The first compound is hexane but the second one is 2-methyl pentane. It is also known as iso-hexane due to its unique Y-shape at the end.
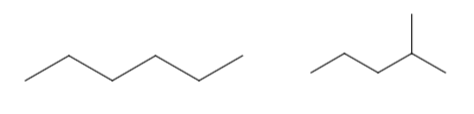
Positional Isomerism
Positional Isomerism
Positional isomerism occurs due to the difference in the position occupied by substituent atom or group or the unsaturation in the chain. In other words, when position of functional group with respect to the main chain changes, it is position isomerism.
This is how \(\ce{C6H19OH}\) exhibits positional isomerism. The first compound is hexanol, the second one is hexan-2-ol, and the third one is hexan-3-ol.
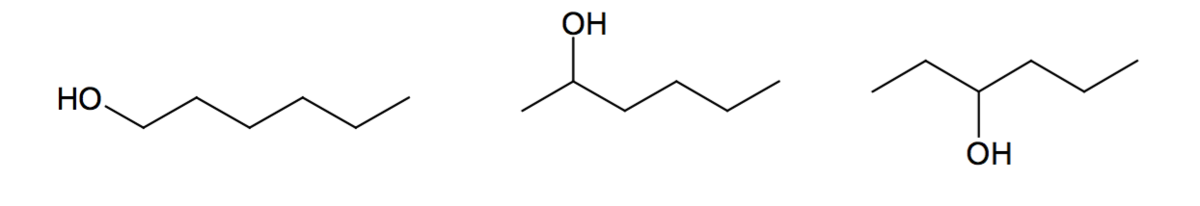
Functional Group Isomerism
Functional Isomerism
Functional isomerism arises due to the presence of odd different functional groups but the chemical formula remains the same. In other words, when a compound can have two different stuctures but the same chemical formula, it exhibits functional isomerism.
Structural isomers having different functional group:
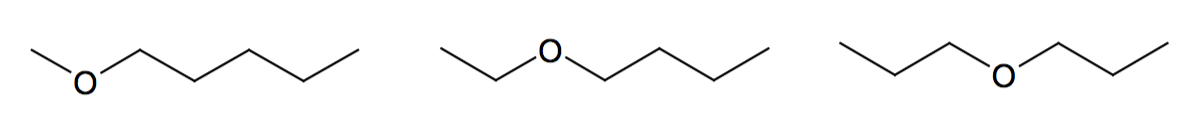
Alcohols and ethers
-OH hydroxyl, -O- ether functional groups
Ketones and aldehydes -CHO aldehyde, >O= carbonyl functional groups

Carboxylic acids and Esters -COOH carboxyl, O>C=O ester functional group

Ring chain isomers are also considered as functional group isomers.
Metamerism
Metamerism
This type of isomerism arises due to the unequal distribution of carbon atoms on either side of the functional group. In other words, when different alkyl groups are attached to a functional group in a compound, it exhibits metamerism.
Tautomerism
Tautomerism
Tautomerism is a special kind of functional isomerism. In this case two isomers are in a stable dynamic equilibrium; that is, they can alter their structure depending on the needs.
Tautomerism is the phenomenon in which two structural isomers differ in the relative positions of their atoms and are spontaneously interconvertiable and can exist in dynamic equilibrium. The two forms in tautomeric equilibrium are called tautomers of each other. The interconvertibility of tautomers is a chemical reaction which involves making and breaking of bonds.
Example:
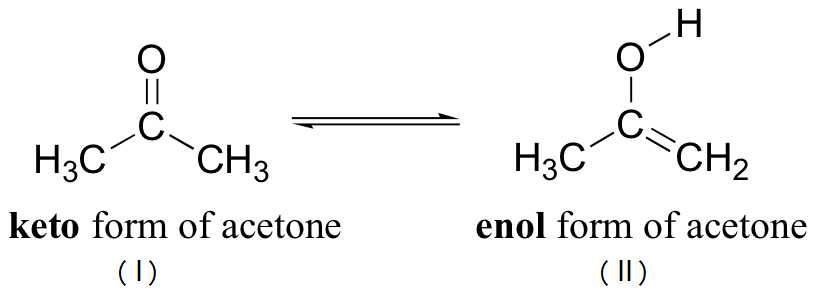
Tautomers (I) and (II) are structural isomers that are formally related only by the shift of a hydrogen atom and one or more \(\pi\)-bonds. Tautomerism, thus in a single way, can also be defined as follows: type of isomerism occuring by the migration of atom (generally acidic hydrogen from carbon to electro negative atom and vice-versa), and the movement of a double bond is called tautomerism. Tautomers are true isomers and either of the individual tautomeric forms may be isolated.

