Acid/Base Equilibrium
Acids and bases have a chemical equilibrium in solution. At chemical equilibrium, the products and reactants have reached a state of balance. Reactions may still be taking place within the sample, but the forward and reverse reactions are taking place at the same rate, so the concentrations of the products and reactants are not changing with time.
Concepts related to acid base equilibrium include designing buffers and plotting pH curves.
Additionally, determining the acid base equilibrium can help in predicting the products of a reaction and the relative concentration of the products, as well as aiding in the identification of the weaker acid and base.
Weak acids and bases are common in nature. For example, many insects of the order hymenoptera, including bees, wasps, and ants, use a weak acid (formic acid) as a weapon when they bite or sting. As a result, many animals avoid contact with these insects. In order for the weaponized acid to be effective, it must produce enough hydrogen ions to wound the bee's enemy, but not enough to dissolve the bee's stinger and organs.
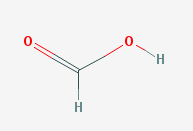 Formic acid
Formic acid
This defense occasionally backfires, as other animals have adapted their own pH in response to formic acid. Anteaters, a species well known for feasting on anthills, do not produce the hydrochloric acid (a strong acid) that most animals rely on for digestion. The anteaters are attracted to the ants, because they need the formic acid to break up their stomach contents.
 Giant Anteater [1]
Giant Anteater [1]
Contents
Defining Strong and Weak
A strong Arrhenius acid dissociates completely in aqueous solution to form \(\ce{H^+}\) ions.
A strong Arrhenius base dissociates completely in aqueous solution to form \(\ce{OH^-}\) ions.An acid showing 100% dissociation in a solution is considered a very very strong acid.
Similarly, a base showing 100% dissociation in a solution is considered a very very strong base.
\(\ce{HCl}\) is considered a strong acid, because it readily and completely dissociates, making \(\ce{H^+}\) ions, as illustrated by the following chemical equation:
\[\ce{HCl_{aq} + H_2O_{l} \rightarrow H_3O^{+}_{aq} + Cl^{-}_{aq}}. \]
\(\ce{NaOH}\) is considered a strong base, because it dissociates completely, forming hydroxide ions \((\ce{OH^-})\). The reaction proceeds as follows:
\[\ce{NaOH_{aq} \rightarrow Na^{+}_{aq} + OH^{-}_{aq}}. \]
Common strong acids and strong bases are listed below.
| Strong Acids | Chemical Structure |
| Hydrochloric Acid | \(\ce{HCl}\) |
| Hydrobromic Acid | \(\ce{HBr}\) |
| Hydroiodic Acid | \(\ce{HI}\) |
| Nitric Acid | \(\ce{HNO3}\) |
| Perchloric Acid | \(\ce{HClO_4}\) |
| Sulfuric Acid | \(\ce{H_2SO_4}\) |
| Strong Bases | Chemical Structure |
| Lithium Hydroxide | \(\ce{LiOH}\) |
| Sodium Hydroxide | \(\ce{NaOH}\) |
| Potassium Hydroxide | \(\ce{KOH}\) |
| Calcium Hydroxide | \(\ce{Ca(OH)2}\) |
| Barium Hydroxide | \(\ce{Ba(OH)_2}\) |
True or false?
A solution containing a strong acid will always burn your skin if you spill it on yourself.
A solution containing a strong base will always burn your skin if you spill it on yourself.
Weak acids and bases do not dissociate completely in aqueous solution.
Here is the general format for weak acid dissociation:
\[\ce{HA_{aq} + H_2O \rightleftharpoons H_3O_{aq}^{+} + A_{aq}^{-}}. \]
Notice the double arrows, \(\rightleftharpoons\), which mean that as the acids is dissociating, it is also re-combining. The reaction can proceed either forward or in reverse, depending on the environmental conditions.
Conjugate Acid-Base Pairs
In aqueous solutions, the reaction between an acid and a base can be thought of as the transfer of a proton from the acid to the base.
\[\ce{HBr + H_2O \rightarrow Br^- + H_3O^+}\]
Note that the hydrogen from \(\ce{HBr}\) (the acid) has moved to \(\ce{H2O}\) (the base), forming the hydronium ion, \(\ce{H_3O^+}\). Water and hydronium are called a conjugate acid-base pair.
Identify the conjugate acid of \(\ce{NH_3}\) in the following chemical reaction:
\[\ce{H_2O + NH_3 \rightarrow NH_{4}^{+} + OH^-}.\]
Water is amphoteric, meaning it can act both as a base and as an acid, depending on its environment.
Common weak acids and their conjugate bases
| Weak Acid | Chemical Structure | Conjugate Base | Chemical Structure |
| Water | \(\ce{H2O}\) | Hydroxide | \(\ce{OH^-}\) |
| Acetic Acid | \(\ce{CH_3COOH}\) | Acetate | \(\ce{CH_3COO^-}\) |
| Hydrocyanic Acid | \(\ce{HCN}\) | Cyanide | \(\ce{CN^-}\) |
Common weak bases and their conjugate acids
| Weak Base | Chemical Structure | Conjugate Acid | Chemical Structure |
| Water | \(\ce{H2O}\) | Hydronium | \(\ce{H_3O^+}\) |
| Pyridine | \(\ce{C_5H_5N}\) | Pyridinium | \(\ce{C_5H_5NH^+}\) |
| Ammonia | \(\ce{NH_3}\) | Ammonium | \(\ce{NH_4^+}\) |
General Forms
Reactions between acids and bases are very common in nature, and often follow one of the following equations.
\((\text{strong acid}) + (\text{strong base}) \rightarrow (\text{salt}) + (\text{water})\)
\[\ce{HCl + KOH \to KCl + H_2O}\]
\((\text{stronger acid}) + (\text{stronger base}) \rightleftharpoons (\text{weaker acid}) + (\text{weaker base})\)
\[\ce{CH_3CO_2H + H_2O \rightleftharpoons H_3O^+ + CH_3CO_2^-}\]
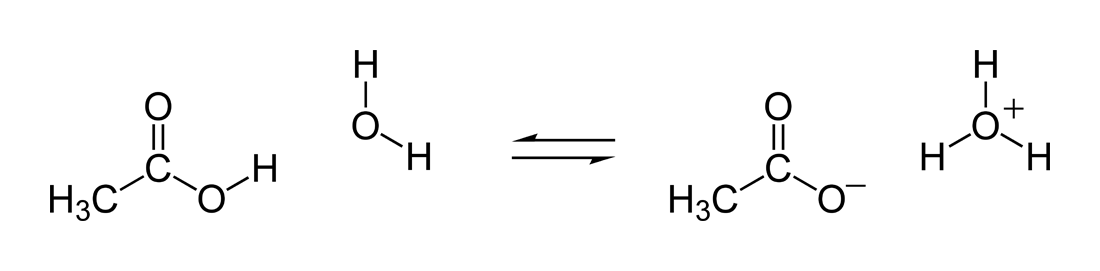 Lewis diagram showing the movement of hydrogen in the reaction between acetic acid and water
Lewis diagram showing the movement of hydrogen in the reaction between acetic acid and water
Acid-base equilibria favor the side of the reaction that has the weaker acid and base. In the above example, the reactants side would be favored and there would be more acetic acid and water in solution, because acetic acid is a weak acid and water is a weak base.
Equilibrium Constants
The equilibrium constants of acids (\(K_a\)) and bases (\(K_b\)) can be used to determine the relative strengths of the compounds. These constants, which are also called dissociation constants or ionization constants, can be used to calculate the percent ionization of an aqueous solution or to determine pH. Additionally, the equilibrium constant for an acid-base reaction can be calculated from the \(K_a\) and \(K_b\) values for the reactants.
where \([\ce{HA}]\) is the concentration of the acid, \([\ce{A^-}]\) is the concentration of the conjugate base, and \([\ce{H_3O^+}]\) is the concentration of hydronium.\[K_a = \dfrac{[\ce{H_3O^+}][\ce{A^-}]}{[\ce{HA}]},\]
where \([\ce{B}]\) is the concentration of the base, \(\ce{BH^+}\) is the concentration of the conjugate acid, and \(\ce{OH^-}\) is the concentration of hydroxide.\[K_b = \dfrac{[\ce{BH^+}][\ce{OH^-}]}{[\ce{B}]},\]
References
- Flores, F. Oso hormiguero (Myrmecophaga tridactyla). Retrieved from https://www.flickr.com/photos/ferjflores/8696742551/