Alkanes, Alkenes, Alkynes
Introduction to Organic Chemistry
Organic chemistry is the study of carbon compounds, so the study of organic chemistry is important because all living things are based on carbon compounds. Carbon is unique in that it can form up to four bonds in a compound, so they can easily bond with other carbon atoms, forming long chains or rings. In addition, the type of bonding in organic compounds is almost always covalent. Organic compounds don't dissociate in solutions because there are no ionic bonds, and therefore organic compounds are poor conductors of electricity and do not behave as electrolytes in solution. Generally, the larger and more complicated the organic substance, the higher its boiling and melting points.
Why is silicon, another element in group 14 of the periodic table, unable to make the great variety of molecules that carbon atoms can?
Silicon can make large molecules called silicones; however, the silicon-silicon bond is much weaker than the carbon-carbon bond, especially with respect to the silicon-oxygen bond. \(_\square\)
So carbon can have four bonds, but the number of bonds carbon makes per atom can also vary. Just to get the terminology out of the way, we'll be looking at what's known as hydrocarbons, which essentially covers molecules that have only carbon and hydrogen atoms. Even more particularly, we'll study aliphatic compounds, which means the compounds will be open-chained. The details of which will be explained later.
When it comes to Alkanes, Alkenes, and Alkynes, the acidity is in the order of Alkynes > Alkenes > Alkanes. The acidity is mainly due to the increase in the s-character which causes an increase in acidity.
Alkanes
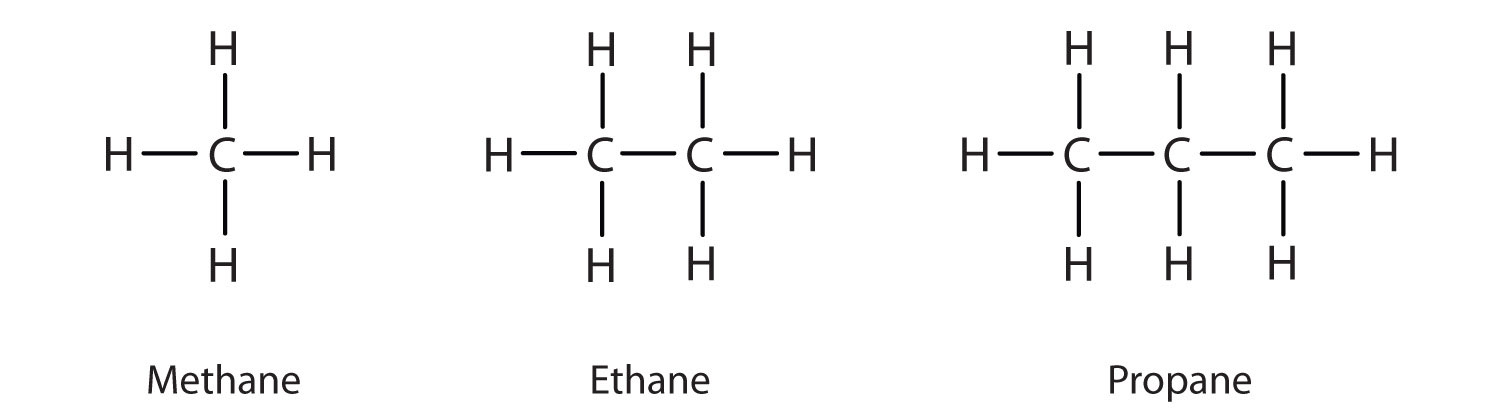
A large and structurally simple class of hydrocarbons includes those substances in which all the carbon-carbon bonds are single bonds. These are called saturated hydrocarbons or alkanes. The simplest alkanes are methane \((\ce{CH_4}),\) ethane \((\ce{C_2H_6}),\) and propane \((\ce{C_3H_8}).\) The alkanes are also called as paraffins. In an alkane, all \(4\) valencies of the carbon atom are satisfied with other hydrogen atoms.
This gives them a general formula : \(\ce{C_{n}H}_{2n+2}.\)
METHANE
Methane gas is the first member of the homologous series of alkanes. The valency of a single carbon atom is satisfied by four hydrogen atoms which form single covalent bonds.
Occurrence
In nature, methane is formed by the microbial activity of organic matter.- Due to decomposition of organic matter in marshy areas (an area of low wetland).
- Natural gas which is a byproduct of petroleum mining contains 70% methane.
- Coal gas too contains 25-40% methane.
Laboratory preparation of methane gas
- \(\color{blue}{\text{Chemicals required:}}\)
You would need the following chemicals:
1) Sodium acetate \((\ce{CH_3COONa})\)
2) Soda lime \((\ce{NaOH + CaO})\) - \(\color{blue}{\text{Chemical reaction:}}\)
\[\ce{CH_3COONa + NaOH ->[\ce{CaO}] {Na}_2CO_3 + CH_4}\] \(\color{blue}{\text{Collection of methane gas:}}\)
Since methane gas is insoluble in water, it is collected by downward displacement of water.Another way of preparation of methane:
\[\ce{{Al}_4C_3 + 12H_2O -> 4Al{(OH)}_3 + 3CH_4}\]Another method of preparation of methane:
\[\ce{CH_3I + 2[H]}\,(\text{from Zn/Cu couple})\ce{-> CH_4 + HI}\]
- \(\color{blue}{\text{Chemicals required:}}\)
Chemical properties
Combustibility
In an environment of excess oxygen, methane burns with a pale blue flame. No soot is formed.\[\ce{CH_4 + 2O_2 -> CO_2 + 2H_2O}\]
In an environment of insufficient oxygen, air pollution can be caused due to release of excess carbon monoxide into the atmosphere.\[\ce{2CH_4 + 3O_2 -> 2CO + 4H_2O}\]
Substitution reaction
In this reaction, the hydrogen atom or atoms in the hydrocarbon are substituted by more reactive atoms such as chlorine, bromine, etc. or group of atoms such as \(\ce{OH, SO_4}\), etc. When hydrogen atoms of an alkane are substituted by chlorine, the reaction is called chlorination.\[\begin{align} \ce {CH_4 + {Cl}_2 &->[\text{Sunlight}] HCl + CH_3Cl} &&(\text{Monochloromethane})\\\\ \ce {CH_4 + 2{Cl}_2 &->[\text{Sunlight}] 2HCl + CH_2{Cl}_2} &&(\text{Dichloromethane})\\\\ \ce {CH_4 + 3{Cl}_2 &->[\text{Sunlight}] 3HCl + CH{Cl}_3} &&(\text{Trichloromethane})\\\\ \ce {CH_4 + 4{Cl}_2 &->[\text{Sunlight}] 4HCl + C{Cl}_4} &&(\text{Tetrachloromethane or carbon tetrachloride}) \end{align}\]
Pyrolysis
The process of decomposition of a hydrocarbon into elements on heating in the absence of air is called pyrolysis.\[\ce{CH_4 ->[1000°\text{C}] C + 2H_2}\]
Catalytic or controlled oxidation
\[\begin{align} \ce{2CH_4 + O_2 ->[\text {Copper - 200°C - 100Atms}] &2CH_3OH}\\\\ \ce{2CH_3OH + O_2 ->[\text {Copper - 200°C - 100Atms}] &2HCHO + 2H_2O}\\\\ \ce{2HCHO + O_2 ->[\text {Copper - 200°C - 100Atms}] &2HCOOH} \end{align}\]
\[\ce{CH_4 + O_2 ->[\text{MoO in 350°C-500°C}] HCHO + H_2O}\]
Examples of reactions with methane
\[\begin{align} \ce {CH_4 + {Cl}_2 &-> HCl + CH_3Cl}\\\\ \ce {CH_3Cl + KOH &-> KCl + CH_3OH} \end{align}\]
\[\begin{align} \ce {CH_4 + {Cl}_2 -> &HCl + CH_3Cl}\\\\ \ce {CH_3Cl + KOH -> &KCl + CH_3OH}\\\\
\ce {CH_3OH + CuO ->[300°\text{C}] &Cu + H_2O + HCHO} \end{align}\]
\[\begin{align} \ce {CH_4 + {Cl}_2 -> &HCl + CH_3Cl}\\\\ \ce {CH_3Cl + KOH -> &KCl + CH_3OH}\\\\ \ce {CH_3OH + 2[O] ->[\ce{K_2{Cr}_2O_7 + H_2SO_4}] &HCOOH + H_2O} \end{align}\]
ETHANE
Laboratory preparation of ethane gas
\(\color{blue}{\text{Chemicals required:}}\)
You would need the following chemicals:
1) Sodium acetate \((\ce{C_2H_5COONa})\)
2) Soda lime \((\ce{NaOH + CaO})\)\(\color{blue}{\text{Chemical reaction:}}\)
\[\ce{C_2H_5COONa + NaOH ->[\ce{CaO}] {Na}_2CO_3 + C_2H_6}\]\(\color{blue}{\text{Collection of ethane gas:}}\)
Since ethane gas is insoluble in water, it is collected by downward displacement of water.Another method of preparation of ethane:
\[\ce{C_2H_5I + 2[H]}\,(\text{from Zn/Cu couple})\ce{-> C_2H_6 + HI}\]
Chemical properties
Combustibility
In an environment of excess oxygen, ethane burns with a pale blue flame. No soot is formed.\[\ce{C_2H_6 + 7O_2 -> 4CO_2 + 6H_2O}\]
In an environment of insufficient oxygen, air pollution can be caused due to release of excess carbon monoxide into the atmosphere.\[\ce{2CH_4 + 5O_2 -> 4CO + 6H_2O}\]
Substitution reaction:
In this reaction, the hydrogen atom or atoms in the hydrocarbon are substituted by more reactive atoms such as chlorine, bromine, etc. or group of atoms such as \(\ce{OH, SO_4}\), etc. When hydrogen atoms of an alkane are substituted by chlorine, the reaction is called chlorination.\[\begin{align} \ce {C_2H_6 + {Cl}_2 &->[\text{Sunlight}] HCl + C_2H_5Cl} &&(\text{Monochloroethane})\\\\ \ce {C_2H_6 + 2{Cl}_2 &->[\text{Sunlight}] 2HCl + C_2H_4{Cl}_2} &&(\text{Dichloroethane})\\\\ \ce {C_2H_6 + 3{Cl}_2 &->[\text{Sunlight}] 3HCl + C_2H_3{Cl}_3} &&(\text{Trichloroethane})\\\\ \ce {C_2H_6 + 4{Cl}_2 &->[\text{Sunlight}] 4HCl + C_2H_2{Cl}_4} &&(\text{Tetrachloroethane})\\\\ \ce {C_2H_6 + 5{Cl}_2 &->[\text{Sunlight}] 5HCl + C_2H{Cl}_5} &&(\text{Pentachloroethane})\\\\ \ce {C_2H_6 + 6{Cl}_2 &->[\text{Sunlight}] 6HCl + C_2{Cl}_6} &&(\text{Hexachloroethane}) \end{align}\]
Catalytic or controlled oxidation:
\[\begin{align} \ce{2C_2H_6 + O_2 &->[\text {Copper - 200°C - 100Atms}] 2C_2H_5OH}\\\\ \ce{2C_2H_5OH + O_2 &->[\text {Copper - 200°C - 100Atms}] 2CH_3CHO + 2H_2O}\\\\ \ce{2CH_3CHO + O_2 &->[\text {Copper - 200°C - 100Atms}] 2CH_3COOH} \end{align}\]
\[\ce{C_2H_6 + O_2 ->[\text{MoO in 350°C-500°C}] CH_3CHO + H_2O}\]
Examples of reactions with ethane
\[\begin{align} \ce {C_2H_6 + {Cl}_2 &-> HCl + C_2H_5Cl}\\\\ \ce {C_2H_5Cl + KOH &-> KCl + C_2H_5OH} \end{align}\]
\[\begin{align} \ce {C_2H_6 + {Cl}_2 -> &HCl + C_2H_5Cl}\\\\ \ce {C_2H_5Cl + KOH -> &KCl + C_2H_5OH}\\\\ \ce {C_2H_5OH + CuO ->[300°\text{C}] &Cu + H_2O + CH_3CHO} \end{align}\]
\[\begin{align} \ce {C_2H_6 + {Cl}_2 -> &HCl + C_2H_5Cl}\\\\ \ce {C_2H_5Cl + KOH -> &KCl + C_2H_5OH}\\\\ \ce {C_2H_5OH + 2[O] ->[\ce{K_2{Cr}_2O_7 + H_2SO_4}] &CH_3COOH + H_2O} \end{align}\]
Alkenes
Alkenes are the unsaturated hydrocarbons in which there is a double bond between two carbon atoms. In these compounds, unsaturation is due to the presence of the double bond.
Alkenes have the general formula \(\ce{C_nH_{2n}},\) where \(n\) is the number of carbon atoms in the molecule. For example,
- if \(n=2,\) the alkene is called as ethene or also as ethylene with the formula \(\ce{C_2H_4};\)
- if \(n=3,\) the alkene would be called as propene or propylene with the formula \(\ce{C_3H_6}.\)

Note: There is no alkene with only \(one\) carbon atom.
ETHENE
Laboratory preparation of ethene gas
- \(\color{blue}{\text{Chemicals required:}}\)
We would require the following chemicals:
1) Ethyl alcohol or ethanol \((\ce{C_2H_5OH})\)
2) Concentrated sulphuric acid \((\ce{H_2SO_4})\) - \(\color{blue}{\text{Chemical reaction:}}\) \[\begin{align} \ce{C_2H_5OH + H_2SO_4 ->[>160°\text{C}]& C_2H_5HSO_4 + H_2O}\\\\ \ce{C_2H_5HSO_4 ->[<160°\text{C} \text{ in excess of }\ce{H_2SO_4}] &C_2H_4 + H_2SO_4} \end{align}\]
- \(\color{blue}{\text{Collection of ethene gas:}}\)
Ethene is insoluble in water and hence it is collected by downward displacement of water. - Another method of preparation of ethene: \[\ce{CH_2{Br}_2 + Zn ->[\text{Ethanol} + \text{Heat}] C_2H_4 + Zn{Br}_2}\]
- Another method of preparation of ethene: \[\ce{C_2H_6 ->[\ce{Al_2O_3 - SiO_2} - 500°\text{C}] C_2H_4 + H_2}\]
- \(\color{blue}{\text{Chemicals required:}}\)
Chemical properties of ethene
- Combustibility:
In excess of air, ethene burns with a pale blue flame.
\[\ce{C_2H_4 + 3O_2 ->[\text{Excess of air}] 2CO_2 + 2H_2O}\]
- Addition reaction:
Ethene is a highly reactive unsaturated hydrocarbon because of the presence of a double bond between two carbon atoms. In the double bond between the carbon atoms, one single bond is weaker. This bond breaks freeing one valence electron of each carbon atom. The free valence electrons then bind to other atoms to form additive compounds in which all valencies of carbon atoms are fully satisfied by single covalent bonds.\[\ce{C_2H_4 + H_2 ->[\ce{Ni-300°C}] C_2H_6}\]
\[\ce{C_2H_4 + {Cl}_2 ->[\ce{C{Cl}_4}] C_2H_4{Cl}_2}\]
\[\ce{C_2H_4 + {Br}_2 ->[\ce{C{Cl}_4}] C_2H_4{Br}_2}\]
\[\ce{C_2H_4 + HCl -> C_2H_5Cl}\]
\[\ce{C_2H_4 + HBr -> C_2H_5Br}\]
\[\ce{C_2H_4 + H_2SO_4 -> C_2H_5HSO_4}\]
\[\ce{C_2H_4 + H_2O + [O] ->[\text{Alkaline }\ce{MnO_4}] C_2H_4{(OH)}_2}\]
- Polymerization:
The process of forming molecules of higher molecular mass by combining similar molecules of lower molecular mass is termed as polymerization.\[\ce{n(H_2C = CH_2) -> -{(H_2C - CH_2)}_n-}\]
- Combustibility:
Examples of reactions with ethene
\[\begin{align} \ce {C_2H_4 + HCl &-> C_2H_5Cl}\\\\ \ce {C_2H_5Cl + KOH &-> KCl + C_2H_5OH} \end{align}\]
\[\begin{align} \ce {C_2H_4 + HCl -> &C_2H_5Cl}\\\\ \ce {C_2H_5Cl + KOH -> &KCl + C_2H_5OH}\\\\ \ce {C_2H_5OH + CuO ->[300°\text{C}] &Cu + H_2O + CH_3CHO} \end{align}\]
\[\begin{align} \ce {C_2H_4 + HCl -> &C_2H_5Cl}\\\\ \ce {C_2H_5Cl + KOH -> &KCl + C_2H_5OH}\\\\ \ce {C_2H_5OH + 2[O] ->[\ce{K_2{Cr}_2O_7 + H_2SO_4}] &CH_3COOH + H_2O} \end{align}\]
Alkynes
Alkynes contain only one triple bond between two adjacent carbon atoms. But if the number of triple bonds is more than one in any compound, the standard IUPAC nomenclature is used. They are commonly called as acetylenes.
They have the general formula \(\ce{C_nH_{2n-2}},\) where \(n\) is the number of carbon atoms in the molecule.
For example, if an alkyne has 2 carbon atoms, then it would be called as ethyne with molecular formula \(\ce{C_2H_2}.\) If an alkyne has 3 carbon atoms, then it would be propyne with formula \(\ce{C_3H_4}.\)

ETHYNE
Laboratory preparation of ethyne gas
- \(\color{blue}{\text{Chemicals required:}}\)
We would require the following chemicals:-
1) Calcium carbide \((\ce{CaH_2})\)
2) Distilled water \((\ce{H_2O})\) - \(\color{blue}{\text{Chemical reaction:}}\)
\[\ce{CaC_2 + 2H_2O -> C_2H_2 + Ca{(OH)}_2}\] - \(\color{blue}{\text{Collection of ethyne gas:}}\)
Ethyne is insoluble in water and hence is collected by downward displacement of water. - Another method of preparation of ethyne:
\[\ce{C_2H_2{Br}_4 + 2Zn ->[\text{Ethanol} + \text{Heat}] C_2H_2 + 2Zn{Br}_2}\] - Another method of preparation of ethyne:
\[\ce{C_2H_2{Br}_2 + 2KOH -> C_2H_2 + 2KBr + 2H_2O}\]
- \(\color{blue}{\text{Chemicals required:}}\)
Chemical properties of ethyne
- Combustibility:
In excess of air, ethene burns with a brilliant white flame.
\[\ce{2C_2H_2 + 5O_2 ->[\text{Excess of air}] 4CO_2 + 2H_2O}\]
- Addition reaction
\[\ce{C_2H_2 + H_2 ->[\ce{Ni-300°C}] C_2H_4 + H_2 ->[\ce{Ni-300°C}] CH_4 }\]
\[\ce{C_2H_2 + {Cl}_2 ->[\ce{C{Cl}_4}] C_2H_2{Cl}_2 + {Cl}_2 ->[\ce{C{Cl}_4}] C_2H_2{Cl}_4}\]
\[\ce{C_2H_2 + {Br}_2 ->[\ce{C{Cl}_4}] C_2H_2{Br}_2 + {Br}_2 ->[\ce{C{Cl}_4}] C_2H_2{Br}_4}\]
\[\ce{C_2H_2 + HCl -> C_2H_3Cl + HCl -> C_2H_4{Cl}_2 }\]
\[\ce{C_2H_2 + HBr -> C_2H_3Br + HBr -> C_2H_4{Br}_2 }\]
\[\ce{C_2H_2 + 2CuCl + 2NH_4OH -> C_2{Cu}_2 + 2NH_4Cl + 2H_2O}\]
\[\ce{C_2H_2 + 2AgNO_3 + 2NH_4OH -> C_2{Ag}_2 + 2NH_4NO_3 + 2H_2O}\]
- Polymerization:
The process of forming molecules of higher molecular mass by combining similar molecules of lower molecular mass is termed as polymerization.\[\ce{3(H_2C_2 = C_2H_2) ->[\text{Red hot silica tube}] C_6H_6}\]
- Combustibility: