Hemoglobin
Hemoglobin (Hb or Hgb) is a molecule found in red blood cells. It carries oxygen from the lungs to the body's tissues and carbon dioxide from the tissues back to the lungs. Hemoglobin is important to both the structure and function of red blood cells.
 Hemoglobin. [1]
Hemoglobin. [1]
Contents
Structure
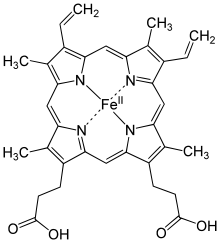 Chemical structure of a heme group
Chemical structure of a heme group
Hemoglobin is made up of four polypeptide chains of two types (two alpha and two beta). Each chain contributes one iron-containing ring structure, or heme group, with one ferrous ion (\(\ce{Fe^{2+}}\)) attached in the center, so each molecule of iron can transport four molecules of \(\ce{O_2}\) gas.
In fetuses and infants, the hemoglobin molecule is made up of two alpha chains and two gamma chains. Fetal hemoglobin has a higher binding affinity for oxygen than adult hemoglobin does, allowing the fetus to transport oxygen from the mother's blood supply and utilize it in utero. After birth, the growth of gamma chains is inhibited. As the infant grows, the gamma chains are gradually replaced by beta chains, forming the adult hemoglobin structure.
When red blood cells are oxygenated, they have a vibrant red color. Which element do the oxygen atoms bind to in hemoglobin to create that color?
Functions
Beyond supplying oxygen to the tissues, hemoglobin serves several important physiological functions.
Hemoglobin is involved in buffering the blood by carrying excess \(\ce{CO_2}\) to the lungs.
Hemoglobin also plays an important role in maintaining the shape of the red blood cells. Normal red blood cells are round with narrow centers; they resemble a donut without a hole in the middle. Sickle-cell-anemia is a hereditary condition caused by a defect in hemoglobin structure. Affected individuals have a single base pair that is different in the gene coding for hemoglobin shape. This single amino acid substitution causes the red blood cells to taper and flatten into a C or sickle shape. The sickled cells polymerize (stick together) more easily, impeding blood flow and causing the cells to rupture.[2]
Generally people with sickle-cell diseases, like sickle-cell anemia, are more prone to infection, so treatments include preventative measures like vaccination, antibiotics, and dietary supplements. More serious treatments include blood transfusions, certain medications, and, in select cases, bone marrow transplants.

Medical Applications
In the 120-day lifespan of red blood cells, sugar molecules attach to the hemoglobin (a process known as glycosylation). People with persistent high blood sugar (those with diabetes) will have higher-than-average levels of the glycosylated hemoglobin called Hemoglobin A1c (\(\ce{HgbA_{1c}}\)). \(\ce{HgbA_{1c}}\) levels are a useful monitoring parameter. While a fasting blood glucose level gives information about a patient's blood sugar at a single point in time, \(\ce{HgbA_{1c}}\) tells the average blood sugar level over the past 3-4 months, giving a more complete picture of a patient's nutrition and overall health and a better idea of whether or not their disease state is controlled.
Binding Affinity and Carbon Monoxide Poisoning
Carbon monoxide (\( \ce{CO}\)) has a binding affinity for hemoglobin that is over 200 times higher than oxygen's. Once carbon monoxide is bound, the hemoglobin is unable to release it and pick up an oxygen molecule. Additionally, hemoglobin that's bound to (\( \ce{CO}\)) (called carboxyhemoglobin) makes it harder for oxygen to dissociate from oxyhemoglobin, meaning that oxygen already in the bloodstream is less accessible to tissues. Carbon monoxide is a colorless, odorless gas. It has many sources, including incomplete combustion of gasoline, defective household furnaces, and cigarette smoking.
Pregnant women are often warned to avoid smoking cigarettes. If a woman does smoke, how high will the levels of carbon monoxide (CO) be in the fetus compared to the levels in the mother?
Changes at High Altitude
Lowlanders visiting a mountainous area often become more tired from the same amount of activity than they would at home. Some even develop altitude sickness.
At sea level, the partial pressure of oxygen is higher than it is at higher elevation. As a result, when gas exchange takes place in the lungs at altitude, the alveolar air, arterial blood, and venous blood all have a lower oxygen content than they would in the same person at sea level if the difference is severe enough, the person experiences hypoxia, a state where the body is no longer meeting its tissues' oxygen needs.
Individuals who move from low elevation to high elevation can acclimatize in several ways, including sustained hyperventilation, increased cardiac output, and increased red blood cell production. Athletes will often train at altitude to improve their oxygen utilization when they compete, as these physiological effects can last for several days after returning to lower elevations.
Acclimatization is short-term, but communities who live at high altitude for multiple generations sometimes develop adaptations to hypoxia. The Aymara of the Andes and the Tibetans are two ethnic groups that live at high altitude, and their genetic adaptations illustrate multiple ways of surviving in a lower-oxygen environment.[3]
The Aymara have much higher erythropoietin levels at altitude, which stimulates red blood cell production, resulting in higher hemoglobin concentrations. This physiological difference leads directly to a higher oxygen saturation compared to populations born at lower elevations. However, this adaptation also has a down side: the Aymara also have high rates of pulmonary hypertension, a type of high blood pressure that can eventually lead to blocked arteries around the lungs and heard.
Tibetans have chronically increased ventilation rates compared to either the Aymara or lowlanders, and their oxygen saturation levels tend to be lower than average. However, they do not suffer from hypoxia. Their body compensates by reducing the pressure in their lungs and increasing capillary density, which allows the tissues to better bind oxygen without increasing hemoglobin.
References
- BerserkerBen, . Hemoglobin_t-r_state_ani. Retrieved August 24, 2016, from https://en.wikipedia.org/wiki/Hemoglobin#/media/File:Hemoglobin_t-r_state_ani.gif
- National Heart, Lung, and Blood Institute, . What is Sickle Cell Disease?. Retrieved from http://www.nhlbi.nih.gov/health/health-topics/topics/sca/
- Beall, C. Tibetan and Andean Patterns of Adaptation to High-Altitude Hypoxia. Human Biology, 72(1), 201-228.