Geometric Isomerism
Geometric isomers are two or more compounds with the same number and types of atoms, and bonds, but which have different geometries for the atoms.
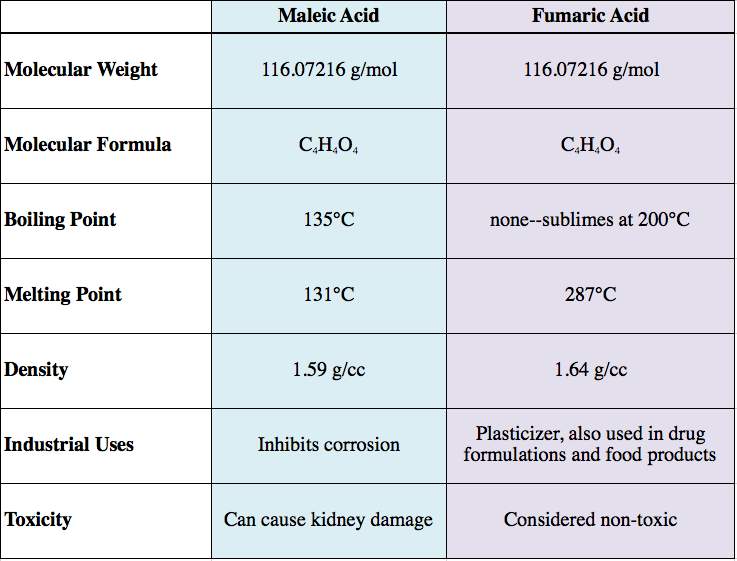
For instance, fumaric acid and maleic acid have the same molecular formula and weight, yet they are not the same molecule. As early as the 1920's researchers sought to understand why fumaric acid killed bacteria, but was not harmful to humans, while maleic acid showed no germicidal activity, but was toxic to humans.[1]
 Fumaric Acid
Fumaric Acid
 Maleic Acid
Maleic Acid
Stereoisomers
Isomers are two molecules that have the same atomic composition, but are not identical. The atoms in the two isomers may be connected in a different order (structural isomerism), or they may be connected in the same way, but have a different orientation is space (stereoisomerism).
Geometric isomerism is a special case of stereoisomerism that follow two requirements:
- There is restricted rotation somewhere in the molecule
- Both atoms involved in the restrictive bond have two different functional groups attached to them
A common example of restricted rotation is a carbon-carbon double bond. These bonds include a pi bond, and it is not energetically favorable to break pi bonds under most conditions.
Isomerism affects the physical properties of the compound. A table comparing properties of maleic acid (the cis isomer) and fumaric acid (the trans isomer) is shown below.
Cis/Trans Naming System
The Cis/Trans naming is the most straightforward system in simple compounds. The longest carbon chain in the molecule is identified, and then the functional groups of interest are identified.
In the cis isomer, the two groups under consideration are on the same side of the double bond (cis means on the same side in Latin).
In the trans isomer, the two groups under consideration are on opposite sides of the double bond (trans means across in Latin).
The two different geometrical isomers of but-2-ene

Note that both of the double-bonded carbons have the same two groups attached to them, \(-H\) and \(-CH_3 \), but the two groups on either one of the double-bonded carbons are different from each other.
While it is easy to inspect the example above and identify the longest carbon chain, the task gets more difficult as side chains and functional groups become more complex.
E/Z Naming System
The official IUPAC naming system uses E/Z notation. There is no specific relationship between cis/trans and E/Z, and the two systems are not interchangeable. E/Z notation uses the Cahn-Ingold-Prelog priority rules and generally is considered more reliable.
The IUPAC name for fumaric acid is (E)-but-2-enedioic acid, and maleic acid is (Z)-but-2-enedioic acid.
References
- Cooper, E., & Edgar, S. (1926). The Biological Significance of cis-trans Isomerism. Biochemical Journal, 20(5), 1060-1070.