Hydrogen Bonds
Hydrogen bonds are an unusually strong form of a dipole-dipole interaction that can occur when hydrogen is bonded to a highly electronegative atom. (The most common are \(\ce{N}\), \(\ce{O}\), and \(\ce{F}\); but \(\ce{S}\) and \(\ce{Cl}\) can also form hydrogen bonds.)
 Hydrogen bonds always form between hydrogen and an electronegative atom.[1]
Compounds with hydrogen bonds have higher-than-predicted boiling points, which helps explain some of the unique behaviors of water--including why liquid water exists on Earth at all! Hydrogen bonding is also important to understanding the properties and behavior of many organic compounds, including alcohols, carboxylic acids, and amines.
Hydrogen bonds always form between hydrogen and an electronegative atom.[1]
Compounds with hydrogen bonds have higher-than-predicted boiling points, which helps explain some of the unique behaviors of water--including why liquid water exists on Earth at all! Hydrogen bonding is also important to understanding the properties and behavior of many organic compounds, including alcohols, carboxylic acids, and amines.
How Hydrogen Bonds Form
As illustrated in the figure below, the highly electronegative atom pulls the electron cloud from the hydrogen atom, which exposes a part of the hydrogen atom's nucleus, giving the atom a partial positive charge. The highly electronegative atoms on nearby molecules can then interact with the hydrogen atom, and since the hydrogen atoms are so small, the dipoles can approach each other more closely than most dipole-dipole interactions.
 Imgur
Imgur
Hydrogen bonds are the strongest of the intermolecular forces. This explains the high boiling point of water compared to similar group 16 compounds. Normally, the boiling point increases with higher molecular mass, due to increasing van der Waals forces.
If hydrogen bonding is NOT considered, which of the following compounds would you expect to have the highest boiling point:
\[\ce{HF}, ~~\ce{HCl}, ~~\ce{HBr}, ~~\ce{or} ~~\ce{HI}?\]
If hydrogen bonding IS considered, which of the following compounds would you expect to have the highest boiling point:
\[\ce{HF}, ~~\ce{HCl}, ~~\ce{HBr}, ~~\text{or} ~~\ce{HI}?\]
Which of the following molecules can form hydrogen bonds with its own kind?
(A) Acetone
(B) Ammonia
(C) Hydrogen chloride
(D) Methane
 For hydrogen bonds to occur, a hydrogen atom directly attached to an \(\text{N}\), \(\text{O}\), or \(\text{F}\) atom must react with another \(\text{N}\), \(\text{O}\), or \(\text{F}\) atom. According to the above structural illustration of the given compounds, only (B) ammonia contains a hydrogen atom directly attached to an \(\text{N}\), \(\text{O}\), or \(\text{F}\) atom. \(_\square\)
For hydrogen bonds to occur, a hydrogen atom directly attached to an \(\text{N}\), \(\text{O}\), or \(\text{F}\) atom must react with another \(\text{N}\), \(\text{O}\), or \(\text{F}\) atom. According to the above structural illustration of the given compounds, only (B) ammonia contains a hydrogen atom directly attached to an \(\text{N}\), \(\text{O}\), or \(\text{F}\) atom. \(_\square\)
In the above example, why doesn't acetone form hydrogen bonds with itself? Couldn't the hydrogen from a \(\ce{CH_3}\) group attach to the oxygen of another acetone molecule?
In acetone, all the hydrogens are attached to carbon, rather than to oxygen, nitrogen, or another highly electronegative element. Since carbon and hydrogen have roughly the same electronegativity, the electrons distribute more evenly in the bond than they do in a hydrogen-oxygen bond, for example. Hydrogen bonds require that hydrogen be bonded directly to an electronegative element, leaving a large partial positive charge on the hydrogen that can attract another electronegative species. \(_\square\)
Which of these compounds can form hydrogen bonds with itself?
Water: How Hydrogen Bonds Make Life on Earth Possible
 Plots showing the boiling points of covalent hydrides for groups 16 and 17.[2]
Plots showing the boiling points of covalent hydrides for groups 16 and 17.[2]
Boiling point tends to increase as the molecular weight of the compound increases. Notice the general trend for the halides of group 16 and 17, ignoring the first point in each line. The boiling point increases in a near-linear fashion moving from the lightest atom in a group to the heaviest. The compounds that form hydrogen bonds (\(\ce{HF} \text{ and } \ce{H_2O}\)) do not follow the pattern. They have significantly higher boiling points, despite having the smallest molecular weights of the hydrides in their respective groups. This anomaly is a direct result of hydrogen bonding. If the trend line for the rest of group 16 were extrapolated, the boiling point for water would be around \(-130^\circ\text{C}\) instead of \(100^\circ\text{C}.\)
Each molecule of water has two hydrogen atoms and two pairs of unshared electrons, allowing it to participate in four hydrogen bonds. The tetrahedral electron configuration of the water molecules causes the hydrogen bonds on each molecule to be spatially separated from the other bonds on that molecule, leading to a very loose packing of molecules in the typical crystal structure of ice. As a result, the density of solid ice is lower than the density of liquid water.
When a lake freezes over in the winter, ice layers form on the top of the lake first because the ice is less dense. The liquid water underneath the surface maintains a stable temperature as the top layers undergo this phase change, allowing the plant, animal, insect, and microbial life inside the lake to survive the winter. If ice were denser than water and sunk to the bottom instead, the lake would eventually freeze solid, guaranteeing a short life for the pond critters and a gruesome sight by spring.
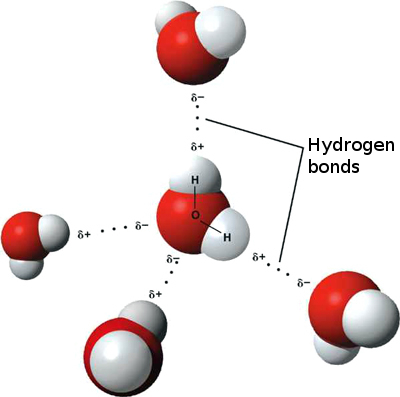 A 3-D model of hydrogen bonding between water molecules. Hydrogen bonds are usually noted by dotted lines.[3]
A 3-D model of hydrogen bonding between water molecules. Hydrogen bonds are usually noted by dotted lines.[3]
References
- Baaden, M. Dynamic water hydrogen-bond network using HyperBalls. Retrieved from https://www.youtube.com/watch?v=pBK22hN7cIM
- Jkwchui, . Boiling Points Chalcogen Halogen. Retrieved from https://commons.wikimedia.org/wiki/File:Boiling-points_Chalcogen-Halogen.svg
- Porto, A. 3D_model_hydrogen_bonds_in_water-es.jpg. Retrieved from https://commons.wikimedia.org/wiki/File:3D_model_hydrogen_bonds_in_water-es.jpg