Inductive Effect, Electromeric Effect, Resonance Effects, and Hyperconjugation
Electronic factors that influence organic reactions include the inductive effect, electromeric effect, resonance effects, and hyperconjugation. These electronic factors involve organic molecules, most of which are made from a combination of the following six elements: carbon, hydrogen, nitrogen, oxygen, phosphorus, and sulfur (known collectively as CHNOPS). Yet, the limited number of building blocks does not prevent organic compounds from taking on diverse properties in their physical characteristics and chemical reactivity. The subtle differentiation of various compounds in organic chemistry is essential for the biological functions of the molecules and creates a wide variety of reactions.
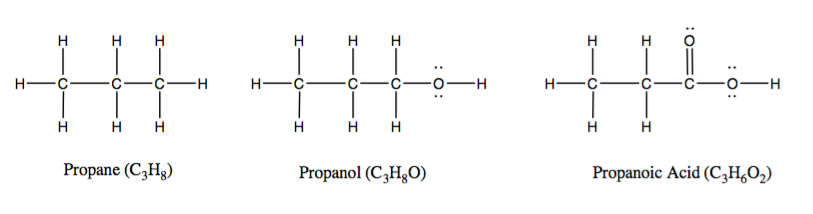
Part of this variety in organic chemistry stems from differences in electron behavior when elements other than carbon and hydrogen participate in molecular bonds. For example, the three compounds pictured above have similar formula units and structures, but react very differently from one another because of these electronic factors. Varying electronegativity can cause delocalization effects, where the electron cloud for a given bond expands to more than two atoms within the molecule.
Contents
Polarity of Organic Molecules
Partial polarity within a molecule leads to electron transfer among the atoms in a molecule, leading to different behavior than what would be expected in a non-polar version of the compound, where no sections were electron-rich or electron-deficient.
Saturated hydrocarbons are nonreactive because there is no polarity in C-C bond and practically no polarity in C-H bonds. Carbon and hydrogen are almost identical in electronegativity, so the electrons involved in a bond between the two atoms are equally attracted to each nucleus and spend roughly the same amount of time orbiting one as the other.
Electron density is evenly distributed between the two atoms in a non-polar bond, which prevents charged species from attacking or altering the bond. In contrast, charged species (electrophiles and nucleophiles) react with polar organic molecules because there is an imbalance in electron density or polarity.
Elements with higher electronegativity, including oxygen and the halide group, can change the electron density around an organic molecule and make the molecule more reactive.
Electronic effects complicate chemical reactions, and they can stabilize a molecule, make a compound less volatile, make a molecule more likely to react in a desired fashion, or affect the acidity or basicity. Understanding the factors involved in electronic imbalance is vital for understanding the underlying mechanisms of a chemical reaction, predicting the products of a reaction and predicting organic molecules' behavior.
Examples of Electronic Effects
The Inductive Effect
Resonance
The Mesomeric Effect
Electromeric Effect
Hyperconjugation
Inductive Effect
The inductive effect is a permanent state of polarization. The electron density in a \(\sigma\) bond between two unlike atoms is not uniform. The electron density is more dense toward the more electronegative of the two atoms.
The inductive effect is a distance-dependent phenomenon:
\[C^{\delta+}-X^{\delta-}\]
The atom \(X\) above acquires a slightly negative charge \((\delta-),\) and the carbon atom a slightly positive charge \((\delta+),\) which means the bond is polarized:
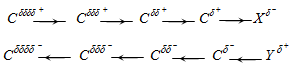
If the electronegative atom \(X\) is connected to a chain of carbon atoms, then the positive charge is relayed to the other carbon atoms. \(C_{1}\), with its positive \(\delta\) charge, exerts a pull on the electrons of \(C_2\), but the pull is weaker than it is between \(X\) on \(C_1\). The effect rapidly dies out and is usually not significant after the \(2^\text{nd}\) carbon atom, or at most the \(3^\text{rd}.\)
The inductive effect is permanent, but relatively weak, and can be easily overshadowed by the electronic effects discussed later.
There are two categories of inductive effects: the electron-withdrawing (-I effect) and the electron-releasing (+I effect). The latter is also called the electron-donating effect. In the image above, \(X\) is electron-withdrawing and \(Y\) is electron-donating.
These relative inductive effects are measured with reference to hydrogen:
\[\text{NO}_2> \text{COOH}> \text{F}> \text{Cl}> \text{Br}> \text{I}> \text{OR}> \text{OH}> \text{C}_6\text{H}_5\text{(Benzene)}> \textbf{H} > \text{Me}_3\text{C}^-> \text{Me}_2\text{CH}^-> {\text{MeCH}_2}^-> {\text{CH}_3}^-.\]
\(\)
-I effect:
The -I effect is seen around a more electronegative atom or group, and electron density is higher there than elsewhere in the molecule. Electron-withdrawing groups include halogen, nitro \((-\text{NO}_2),\) cyano \((-\text{CN}),\) carboxy \((-\text{COOH}),\) ester \((-\text{COOR}),\) and aryloxy \((-\text{OAr}).\)
\(\)
+I effect:
The +I effect is observed among the less electronegative atoms of the molecule by electron-releasing (or electron-donating) groups. The alkyl groups are usually considered electron-releasing (or electron-donating) groups.
Resonance
Sometimes, there are several correct Lewis structures for a given molecule. Ozone \((O_3)\) is one example. The compound is a chain of three oxygen atoms, and minimizing the charges while giving each atom an octet of electrons requires that the central oxygen atom form a single bond with one terminal oxygen and a double bond with the other terminal oxygen.
When drawing the Lewis structure, the choice of placement for the double bond is arbitrary, and either choice is equally correct. The multiple correct ways of drawing the Lewis structure are called the resonance forms.
Based on the resonance forms, a beginning chemistry student might wonder if ozone has bonds of two different lengths, since single bonds are generally longer than double bonds. However, the ozone molecule is perfectly symmetrical, with bonds that are the same length. None of the resonance forms represent the true structure of the molecule. Rather, the negative charge of the electrons that would form a double bond are delocalized, or distributed evenly across the three oxygen atoms. The true structure is a composite, with bonds shorter than what would be expected for single bonds, but longer than the expected double bonds.
 The resonance hybrid for ozone is found by identifying the multiple resonance structures for the molecule.
The resonance hybrid for ozone is found by identifying the multiple resonance structures for the molecule.
Thus for \({ O }_{ 3 }\) the two structures (I and II) shown above constitute the canonical structures or resonance structures and their hybrid (i.e. the III structure) represents the structure of \({ O }_{ 3 }\) more accurately. Resonance is represented by a double-headed arrow between the resonance structures, as illustrated above.
(1)

(2)


The resonance hybrid is more stable than its canonical forms, i.e. the actual compound (hybrid) is at a lower energy state than its canonical forms. Resonance stability increases with increased number of resonance structures.
The difference in the experimental and calculated energies is the amount of energy by which the compound is stable. This difference is known as resonance energy or delocalization energy.
All resonance structures are not equivalent. The following rules help determine whether or not a resonance structure will contribute significantly to the hybrid structure.
Rules of Resonance
Rule 1: The most significant resonance contributor has the greatest number of full octets (or if applicable, expanded octets).
Rule 2: The most significant resonance contributor has the fewest atoms with formal charges.
Rule 3: If formal charges cannot be avoided, the most significant resonance contributor has the negative formal charges on the most electronegative atoms, and the positive formal charges on the least electronegative atoms.
Rule 4: The most significant resonance contributor has the greatest number of covalent bonds.
Rule 5: If a pi bond is present, the most significant resonance contributor has this pi bond between atoms of the same row of the periodic table (usually carbon pi bonded to boron, carbon, nitrogen, oxygen, or fluorine).
Rule 6: Aromatic resonance contributors are more significant than resonance contributors that are not aromatic.
Mesomeric Effect
The permanent polarization of a group conjugated with a \(\pi\) bond or a set of alternate \(\pi\) bonds is transmitted through the \(\pi\) electrons of the system, resulting in a different distribution of electrons in the unsaturated chain. This kind of electron distribution in unsaturated compounds conjugated with electron-releasing or withdrawing groups or atoms is called mesomeric effect.
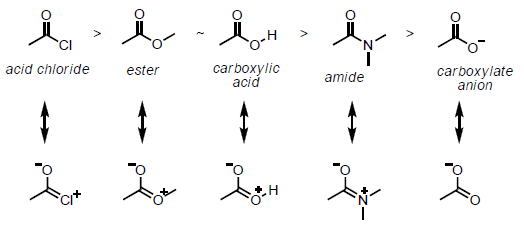
As shown above, a polarity is induced in compounds due to transfer of electrons through \(\pi\) bonds. This effect is a consequence of resonance and is seen in compounds that contain a double bond that is separated from another double bond or a lone pair of electrons by a single bond.
Electromeric Effect
The electromeric effect is an intramolecular movement of electrons from a pi bond to another atom in the molecule due to attack by a reagent. It is temporary and reversible.
There are two distinct types of electromeric effects:
(i) Positive Electromeric Effect (+E effect): In this effect the \(\pi \)-electrons of the multiple bond are transferred to that atom to which the reagent gets attached. For example:

(ii) Negative Electromeric Effect (-E effect): In this effect the \(\pi \)-electrons of the multiple bomd are transferred to that atom to which the attacking reagents do not get attached. For example:

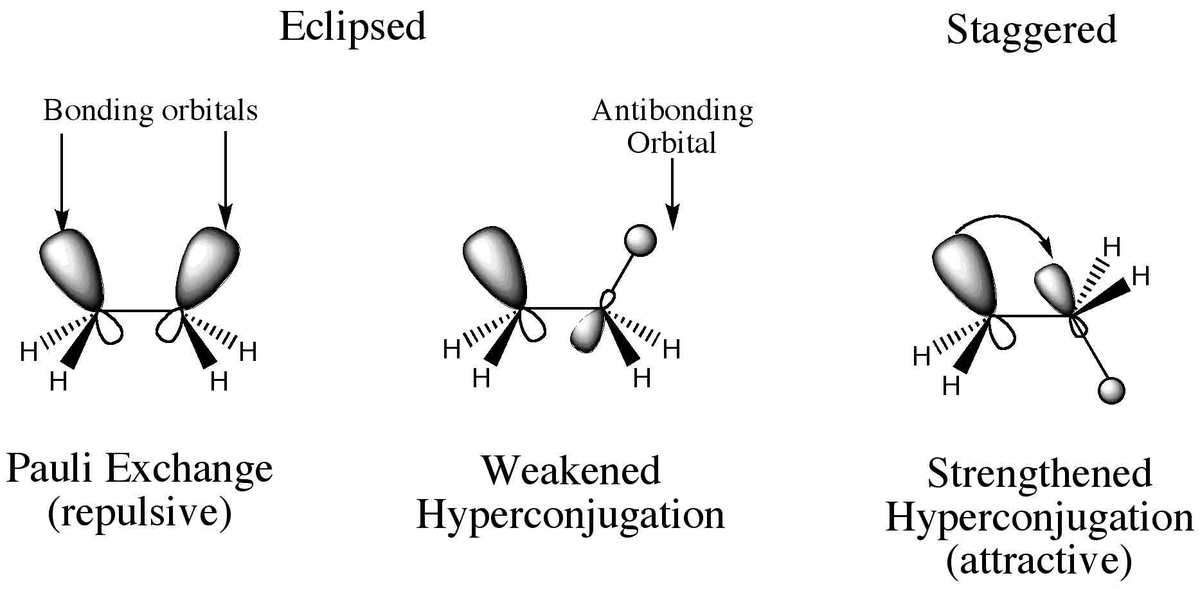
Hyperconjugation
Hyperconjugation helps explain the stability of alkyl radicals. It involves the delocalization of \(\sigma \)-electrons belonging to the C-H bond of the alkyl group attaching to an atom with an unshared \(p\) orbital. The more the hyperconjugative hydrogen, the more is the stability.