Strength of Intermolecular Forces
Intermolecular forces are the attractive and repulsive forces between two distinct compounds or molecules. They include London dispersion forces, dipole interactions, and hydrogen bonds. Intermolecular forces affect many properties of compounds, such as vapor pressure and boiling point.
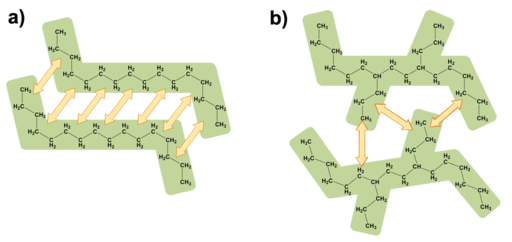 The structure of a compound can influence the formation and strength of intermolecular forces. The branched alkane has fewer opportunities to form London dispersion forces compared to its straight-chain counterpart.[1]
The structure of a compound can influence the formation and strength of intermolecular forces. The branched alkane has fewer opportunities to form London dispersion forces compared to its straight-chain counterpart.[1]
In contrast, intramolecular forces are those that are contained within a single atom or molecule, such as the attraction between an electron and the nucleus it orbits within a carbon atom, or the attraction of an electron to two nuclei in the covalent bond forming a single molecule of carbon monoxide.
Strength of intermolecular forces, listed from weakest to strongest: London dispersion < dipole-dipole < H-bonding
Sometimes, a compound has more than one intermolecular force. For example, water has London dispersion, dipole-dipole, and hydrogen bonds.
 The unit cell for sodium chloride shows ordered, closely-packed ions. Public domain image.
The unit cell for sodium chloride shows ordered, closely-packed ions. Public domain image.

Notice that the sodium chloride crystal has a uniform, predictable pattern with no clearly defined boundaries. In contrast, ice crystals have distinct molecules that are more closely associated. An oxygen atom in a sample of water has a stronger attraction to the two hydrogen atoms making up its own molecule (the intramolecular forces) than to the other hydrogens in the vicinity (the intermolecular forces).
Which of the following options provides the strongest intermolecular force?
Which of the following does not involve an intermolecular force?
References
- Danielchemik, . London forces in alkanes. Retrieved from https://commons.wikimedia.org/wiki/File:London_Forces_in_alkanes.png