Dipole Interactions
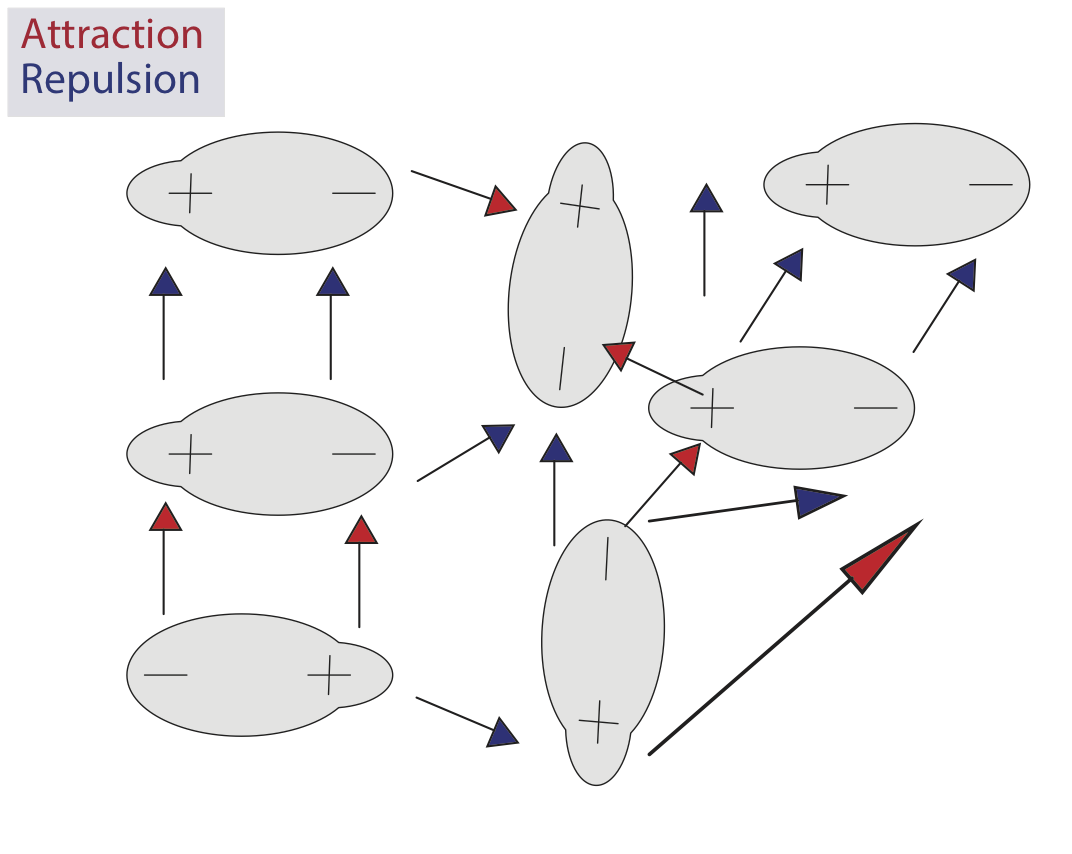
Dipole-dipole interactions are a type of intermolecular force between two molecules that have net dipole moments (asymmetrical charge distributions, where polar molecules develop partial positive and partial negative charges). Molecules tend to align themselves so that the positive end of one dipole is near the negative end of another, and vice versa. When a positive and negative dipole approach each other, it creates an attractive intermolecular interaction whereas two positive dipoles or two negative dipoles will create a repulsive intermolecular interaction. Dipole-dipole interactions and London dispersion forces are collectively referred to as the Van der Waals forces. Dipole-dipole interactions are a type of electrostatic interaction, as are ion-ion interactions and ion-dipole interactions.
 In dipole interactions, opposites attract.
In dipole interactions, opposites attract.
Dipole interactions become more complicated as the number of molecules involved increases. Molecules in a liquid move freely, meaning they are simultaneously creating attractive and repulsive interactions with each other.
Contents
Dipole Moments
Dipole moments (\(\mu\)) are produced by the asymmetrical charge distribution on a polar substance and can be calculated as follows.
\(\mu = \text{Qr}\)
Where \(\text{Q}\) is the partial charge on the bonded atoms (measured in coulombs) and \(\text{r}\) is the distance between the partial charges (measured in meters). It is very effective to predict the ionic or covalent nature of a compound based on value of its dipole moment.
Any diatomic molecule with a polar covalent bond has a dipole moment. In polyatomic molecules with polar covalent bonds, the presence or absence of a net dipole moment depends on the molecular geometry of the molecule, as the individual dipole moments may cancel each other out in a symmetrical molecule. (Molecular geometry can be determined using VSEPR.)
Boiling Points
Intermolecular interactions can affect the physical properties of a compound, such as the boiling point. For a series of compounds with similar molar mass, the boiling point will increase as the dipole moment increases.
| Compound | Molar mass (\(\text{g/mol}\)) | Dipole moment (\(\mu\)) | Boiling point (\(\text{K}\)) |
| cyclopropane | 42 | 0 | 240 |
| dimethyl ether | 46 | 1.3 | 248 |
| acetonitrile | 41 | 3.9 | \(X\) |
Considering the general relationship between dipole moment and boiling point, what is the most reasonable estimate of \(X\)?