Lewis Structure
Gilbert N. Lewis introduced a diagrammatic system to represent the valence shell electrons in an atom. It helps us a lot while doing chemical bonding. He used dots to represent the valence electrons. He used lines to indicate bond between two electrons. Here's an example of carbon, with its valence electrons using Lewis dot structure.

Contents
Representing Electrons
Gilbert Lewis represented the valence electrons using dots. As in the example above, you can see the valence electrons of carbon being represented as dots.
But, when we have to represent a chemical bond between two different atoms, we normally use a different symbol for each atom taking part in the reaction. Let's see an example of a molecule involving two different atoms.
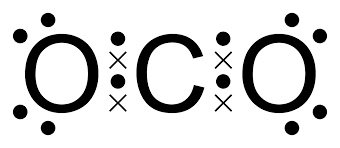
In this compound (carbon dioxide), we find that the electrons of oxygen are represented by dots whereas valence electrons of carbon are represented by a cross.
Note: In case the bond is between two atoms of the same kind, there is no need to use different symbols.
Representing Bonds
We know that the atoms tend to attain the octet state (or duplet state in case of hydrogen) and attain stability. For attaining stability, atoms combine with other atoms, or with the same type of atom. To show the bondings diagrammatically, we need to get to know about the lone pair and bonded pair. As said earlier, (generally) when we represent a chemical bond we draw a line instead of keeping the bonded electrons as such.
Sometimes, not all electrons take part in forming a bond; they are kept away from the bonded pair but are kept around the atom. These un-bonded electrons are generally kept in pairs. Such electrons are called lone pairs.
Bonded pair: There are the pair(s) of electrons which take part in forming a bond. They are normally represented by lines to avoid confusion between them and the lone pairs.
Here's an example of \(\text{NH}_3\) (ammonia), where you can observe the lone pair and the bonded pair:

As said earlier, the bonded pairs are shown as lines. But it's not compulsory to do so; you can keep the bonded pairs as it is. As in the example above, the bonded pairs are not shown as lines. But generally we use lines as it keeps things neat. Here's an example of \(\text{H}_2\text{O}\) (water):
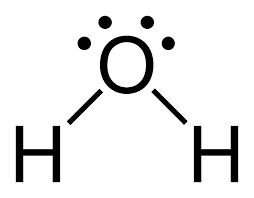
We can observe that the two pairs of electrons have disappeared and in their place a single line is placed, showing a bonded pair. The number of lines shows the number of pairs of bonded electrons.
Calculating Number of Lone Pairs and Bonds
For a neutral atom, we see that the following equation holds good:
\[(\text{no. of bonds}) = (\text{total electrons in a complete valence shell}) - (\text{no. of valence electrons excluding the bonded pair}).\]
Suppose we're given the exercise to find the amount of bonds and lone pairs in the molecule \(\text{NH}_3\). First let's find the amount of total electrons needed for the noble gas configuration. This will be given by \(\sum _{ j=0 }^{k}\alpha_{j}\cdot \beta_{j} \). Where \(\alpha_{j}\) is a constant that will follow from the octet rule. For hydrogen atoms, \(\alpha_{j}\) is equal to 2. For any other atom, \(\alpha_{j}\) is equal to 8. \(\beta_{j}\) is defined as the number of atoms per kind. Let's clarify that a bit by applying it. There is 1 \(\text{N}\) atom. Therefore \(\alpha_{0} = 8\), eight electrons needed per atom, and \(\beta_{0} = 1\), one nitrogen atom. There are 3 \(\text{H}\) atoms. Therefore \(\alpha_{1} = 2\), two electrons needed per atom. And \(\beta_{1} = 3\), three hydrogen atoms.
Thus for \(\text{NH}_3\) we can write:
\(\sum_{ j=0 }^{k}\alpha_{j}\cdot \beta_{j} = 8\cdot1 + 2\cdot3 = 14\)
14 electrons are necessary to obtain the noble gas configuration. Each pair contains two electrons, so there are \( \frac{14}{2}=\boxed{7} \) bonds and lone pairs needed for \(\text{NH}_3\).
Now let's take a look at the amount of valence electrons in \(\text{NH}_3\). \(\text{N}\) has 5 valence electrons in its outer shell. Which means that for "all" nitrogen atoms we have \(1\cdot5=5\) valence electrons in total. \(\text{H}\) has 1 valence electron in its outer shell. Which means that for all hydrogen atoms we have \(3\cdot1=3\) valence electrons. And therefore the total amount of valence electrons is \(1\cdot5 + 3\cdot1 = 8\) valence electrons. This comes down to \(\frac{8}{2} = \boxed{4} \) pairs available (since we're talking about valence electrons in the outer shell).
This means that: \[(\text{no. of bonds}) = 7 - 4 = 3\] And when we check we see that, indeed, \(\text{NH}_3\) has three bonds. The amount of remaining pairs are the lone pairs. They can be calculated by subtracting the no. of bonds from the available bonds. E.g. \(4 - 3 = 1\). So there is one lone pair. And as we can see, that is also true.
Features of Lewis Structures
Each bond is the result of formation of a bond which means sharing of electron pair between a minimum of two atoms.
Each bonded atom contributes at least one electron for sharing, as we can see in all of the above images of Lewis dot structures.
The atoms which share electrons do so because of the need to satisfy octet configuration (In few cases, duplet configuration).
If only one electron pair is shared, the atoms are said to be bonded by a single covalent bond.
Similarly. if two electron pairs are shared, the atoms are said to be bonded by a double covalent bond.
The maximum electron pairs that can be shared is three. If that is the case, the atoms are said to be bonded by a triple covalent bond.