Periodic Table of the Elements
The modern periodic table organizes elements into a grid based on their atomic number. Both the horizontal and vertical positionings of an element within the table give clues as to that element's behavior, making the periodic table a quick and useful reference for predicting how certain elements will react with each other.

Contents
Reading the Periodic Table
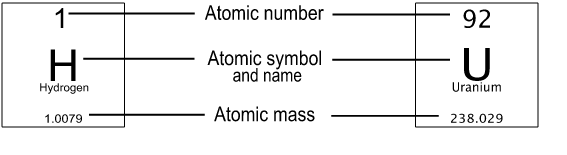
Each box on the table represents one element. Basic information about the element is included on every periodic table, including the following:
- The name of the element
- The one- or two-letter atomic symbol
- The atomic number, which is the number of protons in the atom's nucleus
- The atomic mass in atomic mass units \(\big(\)or AMUs, where one AMU is equal to \(\frac1{12}\) the mass of a carbon-12 atom, or about 1.66 x 10\(^\text{-27}\) kg\(\big).\) (Note: The terms atomic mass and atomic weight are often interchanged, but they do have distinct definitions. Atomic weight is calculated based on the atomic masses and relative abundances of all naturally occurring isotopes of an element.)
History
In the 1800s scientists across Europe were working on the same puzzle: making sense of the patterns of behavior observed in chemical elements and developing a systematic way of organizing those elements.
John Newlands of the United Kingdom, Alexandre Béguyer de Chancourtois of France, and Julius Lothar Meyer and Johann Wolfgang Döbereiner of Germany were among the scientists who contributed to developing a periodic table. They noticed trends and similarities among elements and started dividing them into discrete groups, the best-known of which are Döbereiner's triads and Newlands' octaves.
While specific pieces of these early classifications fit well, no system accommodated all of the approximately 60 known elements.
 Mendeleev's periodic table was first published in the German chemistry journal Zeitschrift fϋr Chemie in 1869.
Mendeleev's periodic table was first published in the German chemistry journal Zeitschrift fϋr Chemie in 1869.
These early chemists faced two hurdles. First, they knew there were more elements to be discovered and incorporated into the periodic table. Second, some of the published information about the elements was known to be wrong. The puzzle had both missing and torn pieces, making it even harder to put together.
Russian chemist Dmitri Mendeleev is often called "the father of the periodic table." In 1869, he published a version with 63 elements arranged by atomic mass, showing that when the elements were arranged that way, certain characteristics were periodically repeated.
Putting the elements in the correct place on the table still sometimes required correcting their atomic mass. Sometimes Mendeleev decided the atomic mass must be wrong because the elements seemed to appear in the wrong order. He placed tellurium before iodine, for example, even though tellurium is heavier. Iodine’s properties are much more similar to those of fluorine, chlorine, and bromine than to oxygen, sulfur, and selenium, and the opposite is true for tellurium.
Mendeleev's table had impressive predictive power. He left blank spaces on the table where he thought undiscovered elements would fit. He predicted several properties for five of those elements, including atomic weight, melting point, density of the solid, and valency. By 1875, three of those elements (gallium, germanium, and scandium) had been discovered by independent researchers in France, Germany, and Sweden, giving further credibility to Mendeleev's periodic table.
Modern Periodic Law
The modern periodic table was devised by Henry Moseley in 1913. Moseley, a young English physicist, carried out research regarding the structure of the atom and came to the conclusion that atomic number, the number of protons in the nucleus, is the fundamental property of an element rather than the atomic mass. This explained some of the inconsistencies Mendeleev was finding. Tellurium is number 52 and iodine is 53, for example.
Moseley's Modern Periodic Law
Properties of the elements are the periodic function of their atomic numbers.
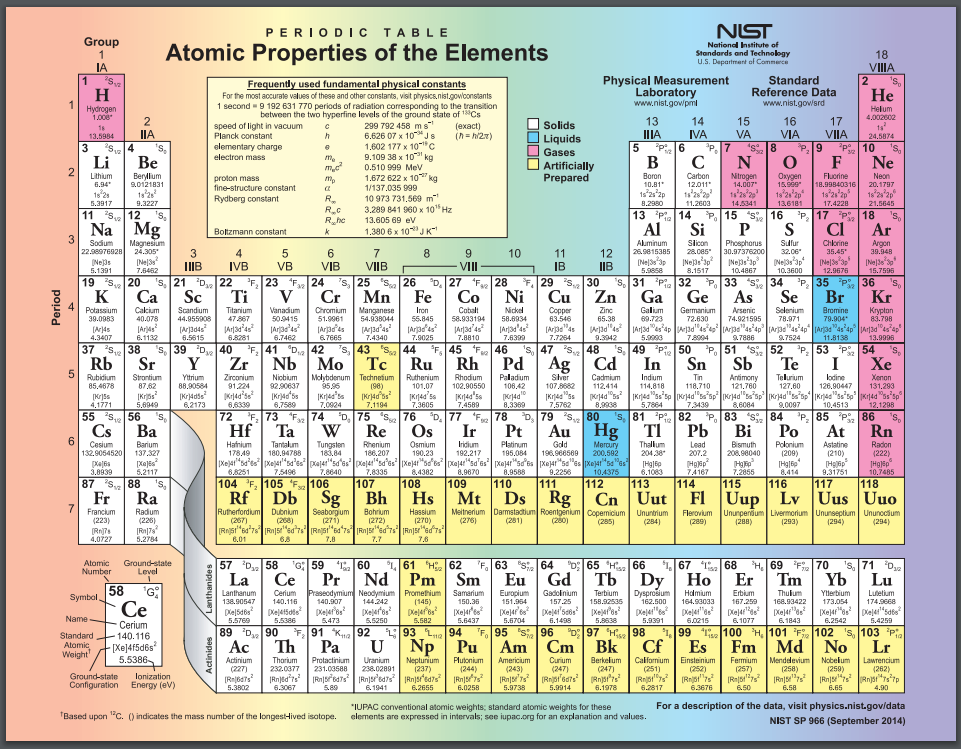 Periodic tables can contain a variety of extra information. This one tells the phase of the pure element at room temperature and notes which elements were created synthetically, rather than discovered in nature. Public domain image from the National Institute of Standards and Technology (NIST).
Periodic tables can contain a variety of extra information. This one tells the phase of the pure element at room temperature and notes which elements were created synthetically, rather than discovered in nature. Public domain image from the National Institute of Standards and Technology (NIST).
Advantages of the modern periodic table include the following:
- The classification of the elements is based on the fundamental property of their atomic number.
- The position of an element is determined by the electronic configuration of the outer valence, which naturally groups elements with similar chemical properties.
- Inert gases, which have completely filled valence shells, are placed at the end of each period.
- It provides a clear demarcation between different kinds of elements such as metals, non-metals, metalloids, transition elements, inert gases, lanthanides, and actinides.
Deficiencies of the modern periodic table include the following:
- The position of hydrogen is unresolved.
- There is no place for lanthanides and actinides in the main body of the table.
- The arrangement is unable to reflect the electronic configuration of many elements in the transition group, lanthanides, and actinides.
Groups
The vertical columns of the table are called groups. Groups have the same electron configuration in their outermost shell or valence. Roman numerals are used to indicate the group number, which is also the number of electrons in the outer valence. There are two sets of groups: A and B. A, the representative elements, have the valence electrons in \(s\) or \(p\) sub-shells. The non-representative elements of group B are the transition metals, which have partially filled \(d\) sub-shells and the lanthanides and actinides, which have partially filled \(f\) sub-shells. Groups with distinct properties the general chemistry student should be familiar with are discussed below:
- Alkali metals, group IA, have many of the characteristic physical properties of metals. Each alkali metal has a single electron in its valence.
- Alkaline earth metals, group IIA, have two electrons in their outer valence.
- Halogens, group VIIA. With seven valence electrons, halogens are close to having a complete octet, making them highly reactive. They can easily strip an electron from a positively charged cation, forming anions with a \(-1\) charge \((\ce{Cl^-}, \ce{F^-},\) etc.\().\)
- Noble gases, group VIII, are also called the inert gases. Their complete valence shell is energetically favorable, making them relatively nonreactive. They have low boiling points and are gases at room temperature.
Periods
The horizontal rows of the table are called periods. There are seven periods, and they are filled sequentially. They represent the principal quantum numbers \(n=1\) to \(n=7\). Arabic numerals are used when referring to the periods.
Periodicity is the repetition of the similar properties of the elements placed in a group separated by certain definite gaps of atomic numbers.
In other words, periodicity is the idea that elements in a group have similar chemical properties because they have the same valence shell electronic configuration. All elements will gain or lose electrons to reach a stable octet, the valence configuration of the noble gases, leading to some general trends across the periodic table.
Going from left to right, electrons are added to the outer valence one at a time. The more electrons there are in the outer shell, the stronger the nuclear attraction. Properties of the vertical groups also factor into periodic trends. Most notably, the number of filled shells increases going down the periodic table. With more electrons between the outer valence and the nucleus, the outer valence is less tightly bound. Together, these two factors explain the following periodic trends:
Atomic radius describes the size of the electrons' orbitals around the nucleus of an atom. The atomic radii decrease from left to right within a period and decrease from bottom to top within a group.
Ionization energy is the energy required to remove an electron from an atom or ion. The closer the electron is to the nucleus, the more difficult it is to remove. Ionization energy increases from left to right within a period and from bottom to top within a group.
Electronegativity measures how strongly an atom is attracted to electrons when it forms a chemical bond. Electronegativity increases from left to right within a period and also from bottom to top within a group.
Electron affinity is a way of measuring how easily an atom can accept an electron. A positive electron affinity indicates that energy is being released when the electron is added. Generalizations for electron affinity can be made for groups within the periodic table by looking at their valence state. For example, the halogens have a high electron affinity because they need one electron to make an octet, while the noble gases have an electron affinity around 0 because they already have an octet.
 Summary of Periodic Trends
Summary of Periodic Trends
Alkaline earth metals have which of the following sets of traits?
Considering its chemical properties and the ions it makes, what is the best location for hydrogen on the periodic table?
Blocks of The Periodic Table
Screening Effect
Lanthanide Contraction
New Elements
In December 2015, the International Union of Pure and Applied Chemistry (IUPAC) announced that four new elements had been discovered and synthesized by laboratories in the United States, Russia, and Japan.
With these new elements, the seventh period of the table is now complete.
The process of naming the elements and giving them two letter symbols is done and the elements are now labeled as Nihonium (Nh, Atomic number 113), Moscovium (Mc, Atomic number 115), Tennesine (Ts, Atomic number 117), and Oganesson (Og, Atomic number 118). New elements can be named after a mythological concept, a mineral, a place, or a scientist. [1]
Other Arrangements
The standard layout of the periodic table does not capture all the patterns and relationships found among the elements. Several alternatives have been proposed to highlight electron configurations or quantum numbers, among other traits. Circular and 3-dimensional versions have been drawn, which eliminate some of the questions regarding the placement of hydrogen and the exclusion of lanthanides and actinides from the main body of the table.

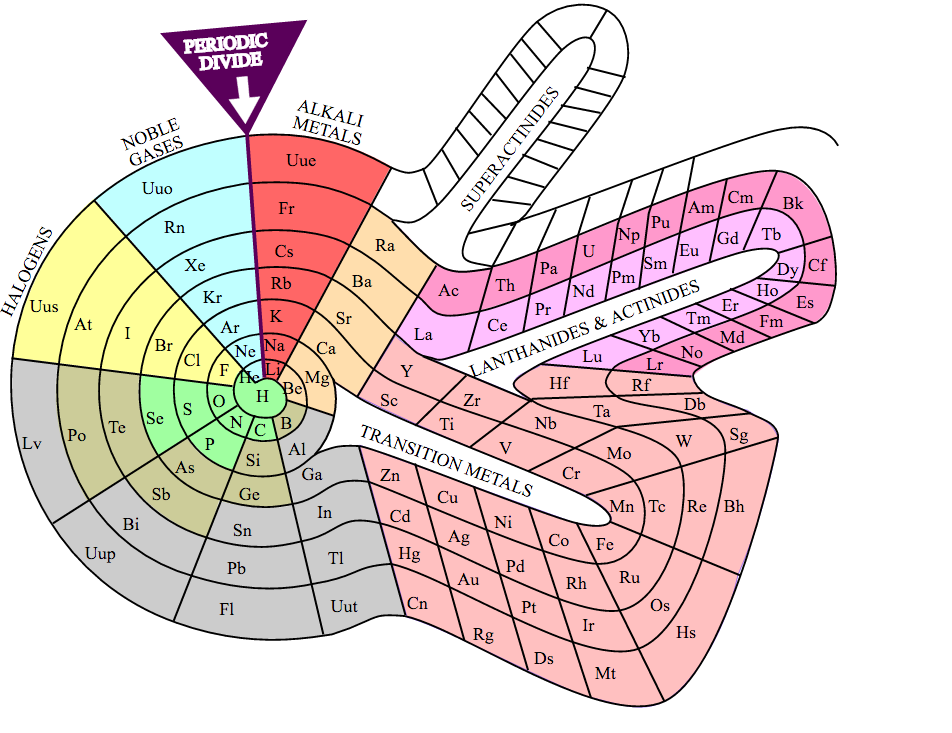 Theodore Benfey's spiral format was first published in the 1960's
Theodore Benfey's spiral format was first published in the 1960's
Interactive Periodic Tables
The periodic table[2] hosted by Los Alamos National Laboratory includes a short article describing the history, properties, and uses of each element.
The University of Nottingham has a website called Periodic Videos[3] that features experiments exploring the properties of the different elements, including plenty of explosions.
References
- IUPAC News, I. Discovery and Assignment of Elements with Atomic Numbers 113, 115, 117 and 118. Retrieved from https://iupac.org/discovery-and-assignment-of-elements-with-atomic-numbers-113-115-117-and-118/
- Los Alamos National Laboratory, U. Periodic Table of Elements. Retrieved from http://periodic.lanl.gov/index.shtml
- University of Nottingham, U. Periodic Videos. Retrieved from http://www.periodicvideos.com