Atoms, Molecules, Elements, Compounds
Atoms are the building blocks of everything you see around: the screen you are looking at, your study table, your books, etc. Such is the amazing power of nature and fundamental nature of these particles. Despite the discovery of sub-particles like electrons, protons and neutrons, an atom continues to remain the fundamental particle because of the fact that it is the smallest unit humans can calculate and model that exhibits the chemical properties of an element.
Chemistry is essentially the study of matter and the changes it undergoes in everyday activities like cooking to more complex processes such as photosynthesis. In essence, the heart of chemistry involves studying changes around our world. Let us discuss some basic ideas of atoms, molecules and the matter they make--elements and compounds.
Contents
Atoms
As stated earlier, an atom is the smallest constituent particle of an element which exhibits the chemical properties of an element and also can take part in a chemical reaction. Atoms are extremely small and their sizes are about an angstrom. \(\big(1\) angstrom \((\text{A}^{\circ})= 10^{-10}\text{ m}.\big)\) Although visualizations are nowadays made by very sophisticated microscopes, no one has actually seen an atom. Initial studies showed that it is spherical in shape. However, the recent quantum mechanical model, which today is widely accepted, suggests that it does not have a fixed shape or structure. (For representative purposes and convenience, we however assume it to be spherical.) This is due to the uncertainty principle, as proven by Werner Von Heisenberg, popularly known as Heisenberg uncertainty principle.
The word "atom" has a Greek origin from the verb "temnein" meaning "to cut." Thus atom means something that is indivisible. When Dalton gave his atomic theory, it was believed that atom was indivisible and hence the name. But later it was found that atoms have been subdivided into simpler parts. These simpler parts are called the subatomic particles. Based on the standard atomic model, there are 12 fermions (having fractional spin), 4 gauge bosons (having complete integer spin), and the Higgs boson.

The fermions include the quarks and the leptons:
Quarks: There are 6 flavors (meaning types, but in the language of quantum mechanics, flavors) quarks and their counterparts in antimatter. But the discussion of antimatter is not a subject here. The quarks, when bound by gluons (read on to find out what they are), form hadrons. The most stable hadrons are protons and neutrons. The smaller the quark, the more mass it has. There are 3 generations of quarks:
- \(1^\text{st}\)-generation Quarks (most stable): These include the up quark and the down quark.
\(\qquad\) Up quark: The up quark has a charge of \(+\frac{2}{3}\) and is the least massive of all the quarks.
\(\qquad\) Down quark: The down quark has a charge of \(-\frac{1}{3}\) and has about the same mass as the up quark.
\(1^\text{st}\)-generation quarks are 1 attometer \(\big(10^{-18}\) meter\(\big)\) in size. The protons and neutrons both have 3 valence quarks.
\(\qquad\) (1 proton) = (2 up quarks) + (1 down quark) + (quark-antiquark pairs). The fractional charges add up to +1.
\(\qquad\) (1 neutron) = (1 up quark) + (2 down quarks) + (quark-antiquark pairs). The fractional charges add up to 0. \[\] \(2^\text{nd}\)-generation Quarks: These include the strange quark and the charm quark. They quickly decay into the \(1^\text{st}\)-generation quarks.
- Strange quark: Has a charge of \(-\frac{1}{3}\). Bigger than charm quark, hence less mass than it. 50 times more massive than the up and down quarks. Strange matter is made up of up, down, and strange quarks and quark-antiquark pairs.
- Charm quark: More massive than the strange quark. Has a charge of \(+\frac{2}{3}\).
\(3^\text{rd}\)-generation Quarks: top quark and bottom quark
- Top quark: Has a charge of \(+\frac{2}{3}\). Quickly decays into other quarks. The particle is not possible to detect. It was discovered by looking at the decay products.
- Bottom quark: Has a charge of \(-\frac{1}{3}\). The most massive of all the quarks. It also decays quickly.
Peggy has his experiment seen.
- \(1^\text{st}\)-generation Quarks (most stable): These include the up quark and the down quark.
Leptons: There are electrons, muons, tauon, electron neutrino, muon neutrino, and tau neutrino. All leptons have a spin of \(\frac{1}{2}\). The leptons that are not neutrinos have a charge of -1. Neutrinos have a charge of 0.
Bosons: Bosons are any particle that follows Bose-Einstein statistics. Bosons in the standard model are photons, gluons, Z bosons, and \(\pm\)W bosons. Bosons "carry" forces. The photon carries electromagnetic radiation. The gluon carries the strong force. ±W bosons and its antiparticle, the Z boson, carry the weak force. All bosons have an integer spin. The Higgs boson has 0 spin and even parity. It creates the Higgs field which stops all particles from moving at 299,792,458 m/s. The amount of interaction is called mass.
Atomic Model
There were many theories on the structure of an atom. Here are some of the most important ones.
J.J. Thomson's Model
Using an ampoule similar to Crooke's but with the perforated anode and a set of equipment that formed an electric field and a magnetic field, Thomson managed to discover the relationship load/mass of the electron.
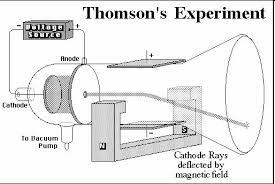
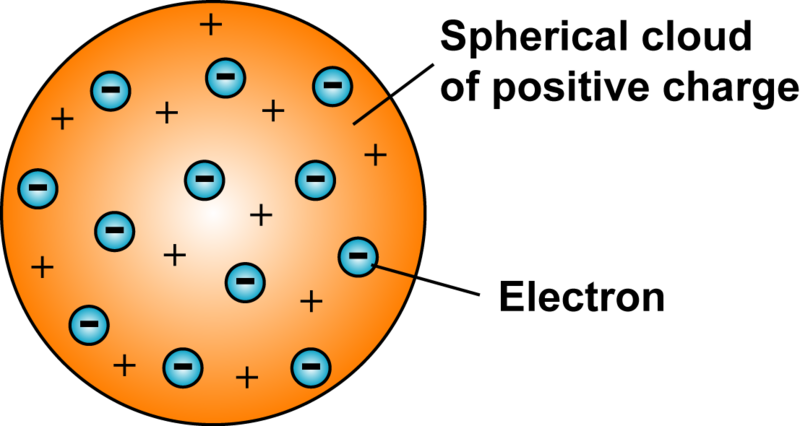
Thomson laid the fundamental concept of the structure of the atom. He referred to it as the plum pudding model, in which the nuts showed the negatively charged particles.
He proposed the following key points:
- An atom consists of a positively charged sphere and the negatively charged particles are embedded in it.
- The negatively charged particles and the positively charged particles are equal in magnitude, i.e. an atom is electrically neutral.
- An important feature of this model is that the mass of the atom is assumed to be uniformly distributed over the atom.
This model had serious drawbacks; it was proved that this was a wrong concept by the next model as it couldn't explain the results of the gold foil experiment carried out by Ernest Rutherford in 1911.
Rutherford's Model
He conducted a very famous experiment, the gold foil experiment or the \(\alpha\)-particle scattering experiment. In this experiment, he bombarded alpha particles on a thin gold foil. He expected that the α-particles would be deflected by the gold atoms, as he believed that the atom was a positively charged sphere. Moreover, he thought that matter was composed of atoms, in which there was no space. Imagine it like a row of marbles, one next to the other. With this idea in mind, all alpha particles should bounce back.
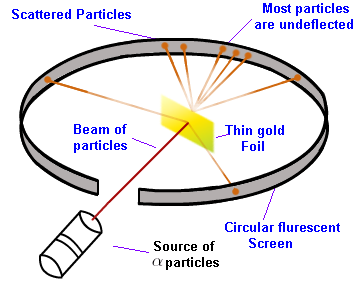
Astonishingly, only one out of every \(12000\) \(\propto\)-particles bounced back. And very few got deflected by some angles. So he concluded as follows:
- The positively charged sphere, the nucleus, is very small and densely packed, situated in the center and most of the space is empty.
- The electrons move around the nucleus in circular orbits with a very high speed.
- A few positively charged \(\alpha\)-particles were deflected. The deflection must be due to enormous repulsive force showing that the positive charge of the atom is not spread throughout the atom as Thomson has presumed. The positive charge has to be concentrated in a small volume. The size of the nucleus is very small, i.e. most of the space in the atom is empty. \((\)One angstrom for the atom, \(10^{-15}\text{ m}\) for the nucleus, which means the nucleus is 100000 times smaller than the atom\(.)\)

But later his second conclusion was not accepted, because if the electrons revolve in a circular motion, they must spend energy and finally will fall into the nucleus, which means the atoms will be unstable. After a few years, Niels Bohr came up with a new model explaining the stability correctly. Although this model got the basics right, there are fundamental problems with it. For one, it doesn't explain why there are only 2 electrons in the K-level.
Bohr's Model
He explained the drawbacks of the previous model. His proposed the following:
- The electrons revolve in discrete orbits, which helps them in overcoming the energy loss.
- They don't radiate energy in these orbits. Hence they are stable.
- Angular momentum is quantized.
What are Orbitals?
Quantum mechanical models are the modern concept to explain the structure of atoms and describe the state of electrons in an atom in terms of probability of finding electrons in the space around the nucleus. This leads to the concept of orbitals.
Orbitals
It is defined as "the three-dimensional region in space around the nucleus where the probability of finding the electron is maximum."
The classical concept of orbits, like planets revolving a sun, for example, is given up in the quantum mechanical description of the atomic world. We only know where there is a high chance that the electron is to be found. We can no longer pinpoint the position of the electron (nor the proton nor the neutron...), but we can define a probability function which gives us a good idea of where it should be. Thus the orbitals are the region where the probability of finding an electron is high. An electron can move anywhere in an atom, even inside the nucleus, or theoretically even at the other side of the universe, but 90% probability is that it is found in a small specific region of space around the nucleus, and this region is nothing but the"orbital."
This idea radically changes our view, to the extent that even Einstein himself doubted the model. He was willing to consider it a correct, yet incomplete model. The fundamental notion that we can only statistically gain information about quantum mechanical properties is a radical thought changer from the deterministic view Newton used to uphold. Even the theory of relativity is utterly deterministic.
However, all experiments conducted so far strengthen the quantum mechanical and we even have strong reasons to believe the great master himself was wrong in his doubts.
Parts of an Atom
Finally, after much debates, Bohr's model was accepted as the universal model. Let's now look into the final structure and the parts of an atom. There are mainly three subatomic particles—the electron, the proton, and the neutron. The protons and the neutrons together form the nucleus and are thus called the nucleons.
The protons are positively charged and carry a charge of \( + 1.6 \times 10^{-19} \text{ C} \), while the neutrons do not have any charge.
The electrons, on the other hand, are negatively charged. They carry a charge of \( - 1.6 \times 10^{-19} \text{ C} \). An atom contains an equal number of protons and electrons, thus making an atom overall electrically neutral.
The neutron is slightly heavier than a proton and they together constitute more than \( 99 \)% of the mass of an atom. This is because the mass of an electron is about \( \frac{1}{1837} \) times the mass of a proton.
According to the quantum mechanical theory, an atom does not have a specific shape or a structure. The positively charged nucleus is at the center surrounded by an electron cloud. Such terminology is used keeping in mind the Heisenberg uncertainty principle and the wave-particle dual nature of electrons.
The negatively charged electron cloud is held close to the nucleus mainly by the attractive electrostatic forces. Well, the cloud isn't that close either. In fact, it is very widespread. The size of the nucleus is estimated to be around \( 10^{-5} \) times that of the entire atom. That means all the protons and neutrons are packed tightly in a sphere whose size is about \(10^{-15} \text{ m}.\) So what keeps the protons in the nucleus together?
They are stronger and short-ranged nuclear forces. To have a rough estimate of how strong they are, just try calculating the Coulomb repulsion force between two protons at that separation. In reality, the separations are much smaller.
Atomic Number
Atomic number is nothing but the number of protons present in the nucleus of an atom. It is denoted by 'Z'. For neutral atoms, it is the number of protons or electrons in an atom.
Atomic Mass Number
An atomic mass number is the sum of numbers of protons and neutrons present in the nucleus of an atom. It is denoted by 'A'.
An element has 11 protons and 12 neutrons in the nucleus of its atom. Find the atomic number (Z) and atomic mass number (A) of that element.
We have
- Atomic number (Z) = Number of protons = 11
- Atomic mass number (A) = Number of protons + Number of neutrons =11 + 12 = 23.
Therefore, the atomic number (Z) and the atomic mass number (A) of that element are 11 and 23, respectively. \(_\square\)
Which of the following is the farthest from the center of an atom?

Molecules
Atoms, in general, seem to be extroverts. They can easily combine with one another and form more complex particles called molecules. Molecules are groups of atoms held together by chemical bonds and possess no net charge. Molecules can simply be defined as the way atoms exist in nature.
Molecules, in general, are the smallest entities that can represent the chemical properties of a compound. It is strange to define a molecule using a compound, and then a compound using the idea of molecules, but they are so interconnected that it is difficult to find independent definitions. (The picture is made clear when a compound is defined.) So, basically, molecules are the basic units of everything—everything that you see around, everything you can think of.
Molecules are formed when two or more atoms react and chemically combine under certain conditions. Molecules can be categorized as homonuclear and hetero nuclear. It is pretty much evident from the names itself.
A homonuclear molecule is formed when the combining atoms are of the same element. For example, two hydrogen atoms combine together and form a stable homonuclear molecule, di-hydrogen \((\text{H}_2)\).
On similar lines, a heteronuclear molecule consists of atoms of different elements. For example, when two atoms of hydrogen and an atom of oxygen chemically combine, they form a stable heteronuclear compound, water \((\text{H}_2\text{O})\).
The molecules can also be classified on the basis of the interaction between the atoms, i.e. the nature of the bond between the atoms. It can be covalent, i.e. there is an overlap of electron cloud of the two interacting atoms and the increased interaction between the overlapping electrons reduces the energy of the system keeping it intact. It can also be ionic wherein one of the atoms loses an electron to become a positively charged ion while the other interacting ion gains an electron to become a negatively charged ion and these ions are held together by electrostatic forces.
Molecules can react with one another to form different molecules and thus different compounds. It is thus molecules that are in general involved in chemistry and chemical reactions, much more than atoms.
Which of the following compounds is not a molecule?
\[\begin{array} &\text{(A) } \ce{H_2O} &&&\text{(B) } \ce{NaF} &&&\text{(C) } \ce{CO_2} &&&\text{(D) } \ce{NH_3} \end{array}\]
The answer is B, and the reason is that compounds with any ionic bond are not molecules.
- In \( \ce{H_2O} \), oxygen atom \(\ce{O}\) has two different single covalent bonds with hydrogen atoms \((\ce{H-O-H}).\) Therefore it's a molecule.
- In \(\ce{CO_2}\), carbon atom \(\ce{C}\) has two different double covalent bonds with oxygen atoms \((\ce{O=C=O}).\) Therefore it's a molecule.
- In \(\ce{NH_3}\), nitrogen atom \(\ce{N}\) has 3 different single covalent bonds with 3 different hydrogen atoms. Therefore it's a molecule as well.
- In \(\ce{NaF}\), sodium atom \(\ce{Na}\) has an ionic bond with fluorine atom \((\ce{Na-F}).\) Therefore it's not a molecule.